RINVOQ® (upadacitinib) Overview
Primary endpoints: §p<0.01; *p<0.001 vs placebo; ‡nominal p<0.05 vs placebo. †First time when primary endpoint measure separated from placebo. ¶Ranked secondary endpoints p<0.05 measured at the same time as the primary endpoint. Clinical Remission does not imply drug free remission.
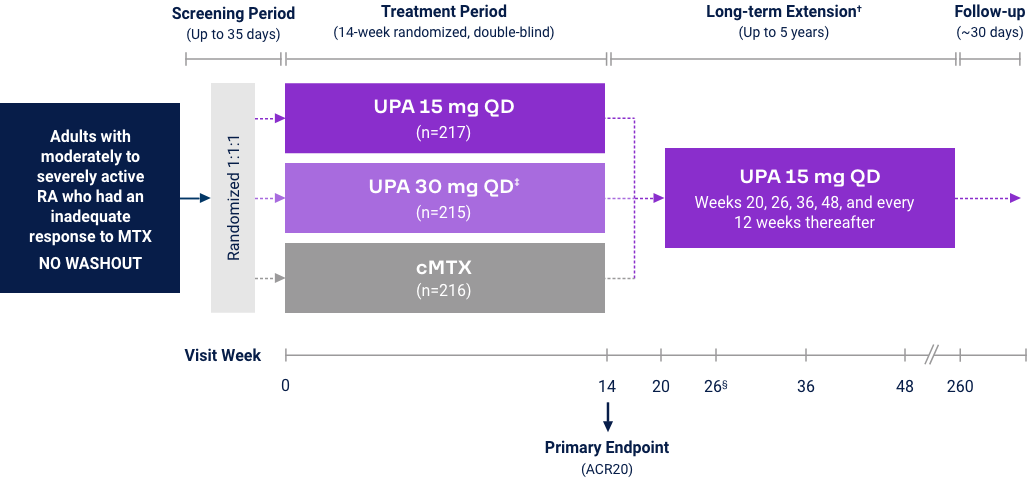
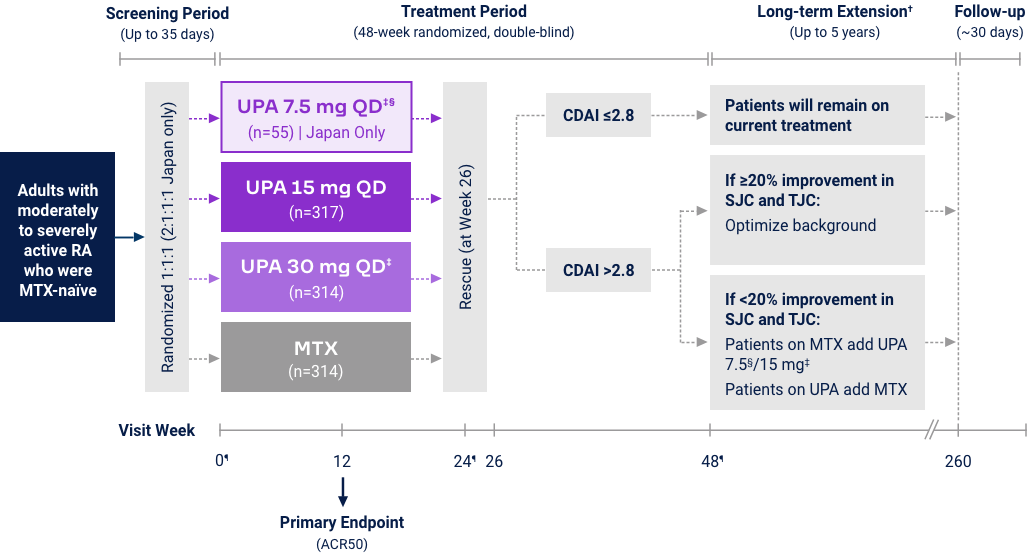
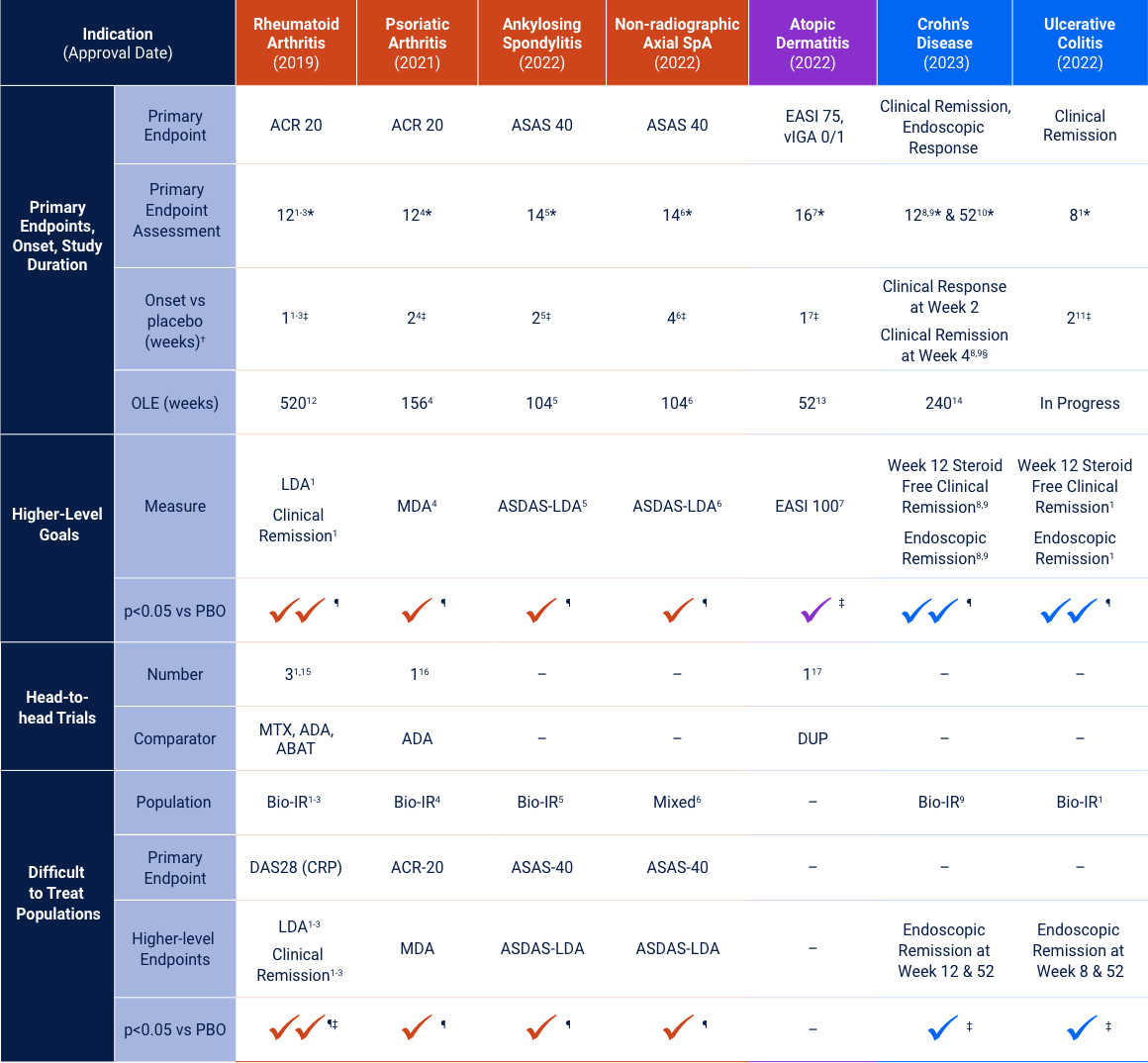
Rheumatoid Arthritis
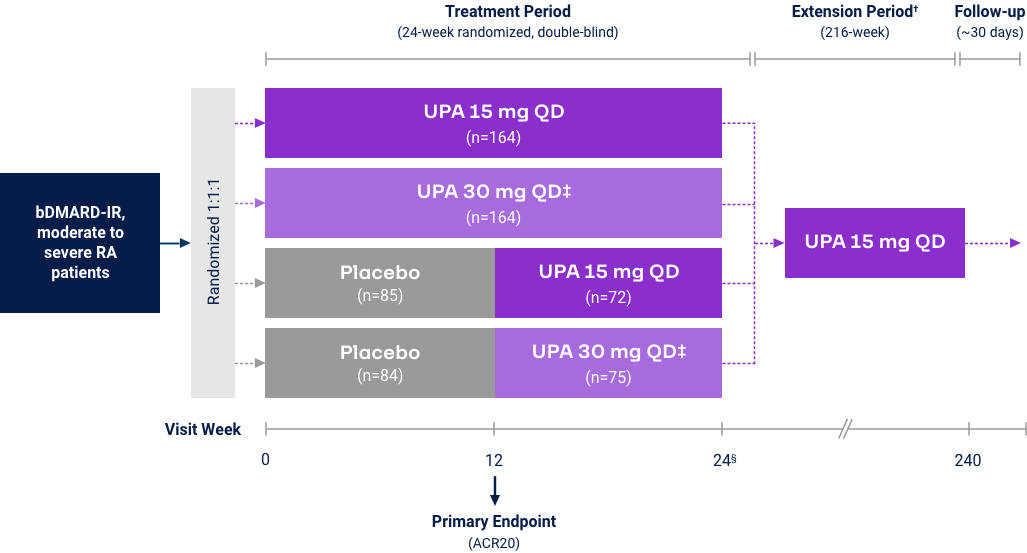
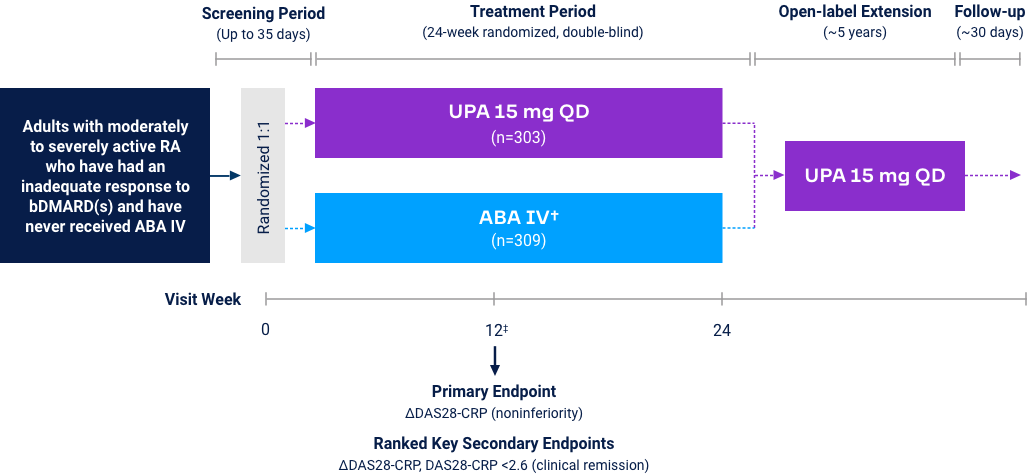
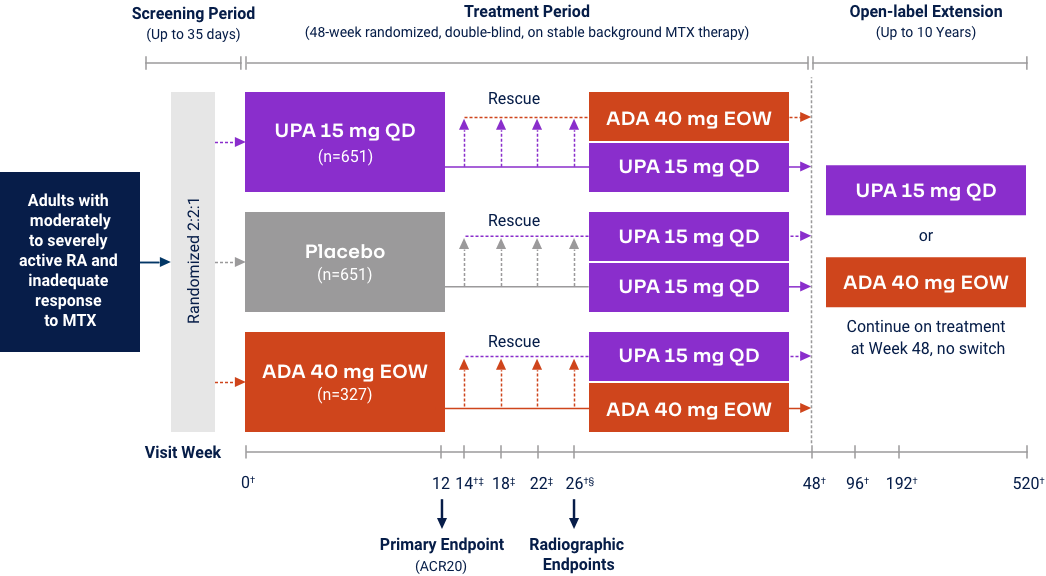
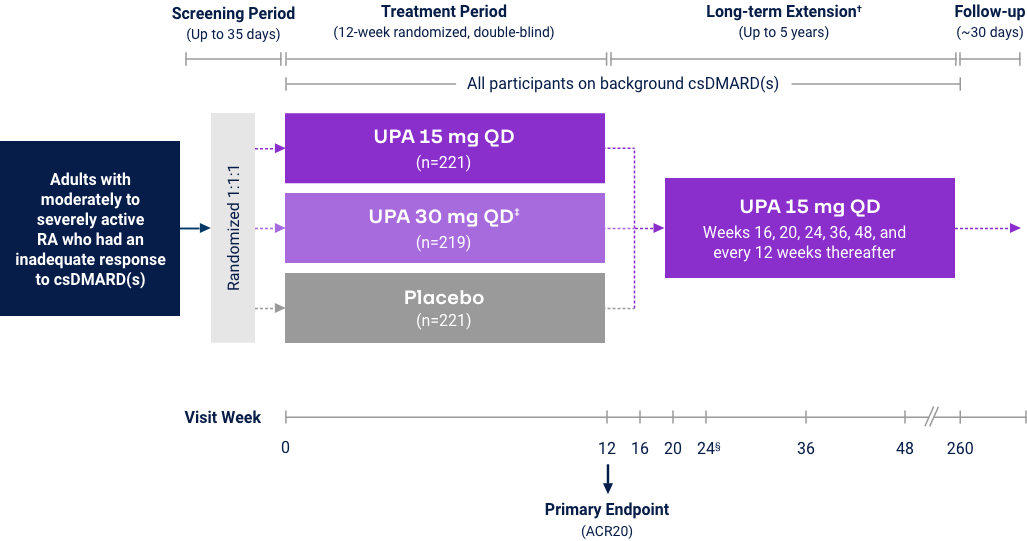
Upadacitinib 15 mg met all primary and ranked secondary endpoints1,15,18-22*
|
(Bio-IR) |
(Bio-IR) |
(MTX-IR) |
(csDMARD-IR) |
(MTX-IR) |
((MTX-naïve) |
||
|---|---|---|---|---|---|---|---|
| Measure at Week 12 | UPA vs PBO | UPA vs ABAT§ | UPA vs PBO | UPA vs ADA | UPA vs PBO | UPA vs cMTX | UPA vs MTX |
| Primary endpoint | |||||||
| ACR20 or ACR50 (EARLY) or ΔDAS28-CRP (CHOICE) |
<0.001 | <0.001 (noninferior) |
<0.001 | N/A | <0.001 | <0.001 | <0.001 |
| Ranked secondary endpoints | |||||||
| ΔDAS28-CRP | <0.001 | See above | <0.001 | N/A | <0.001 | <0.001 | <0.001 |
| ΔHAQ-DI | <0.001 | N/A | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| ΔSF-36 PCS | <0.001 | N/A | <0.001 | N/A | <0.001 | <0.001 | <0.001 |
| CR by DAS28-CRP <2.6 | N/A | <0.001 | <0.001 | N/A | <0.001 | <0.001 | <0.001 |
| LDA by CDAI ≤10 | N/A | N/A | <0.001 | N/A | <0.001 | N/A | N/A |
| ΔMorning joint stiffness duration | N/A | N/A | <0.001 | N/A | <0.001 | <0.001 | N/A |
| ΔFACIT-F | N/A | N/A | <0.001 | N/A | <0.001 | N/A | N/A |
| ΔmTSS at Week 24/26 | N/A | N/A | <0.001 | N/A | N/A | N/A | <0.001 |
| LDA by DAS28-CRP ≤3.2 | <0.001 | N/A | <0.001 | N/A | <0.001 | <0.001 | <0.001 |
| ΔPatient’s pain assessment | N/A | N/A | N/A | ≤0.001 | N/A | N/A | N/A |
| ACR50 | N/A | N/A | N/A | ≤0.001 (noninferior) |
N/A | N/A | N/A |
*Different primary and ranked key secondary endpoints were prespecified for the FDA (shown here) and the European Medicines Agency. †Upadacitinib is only indicated for patients who have had an inadequate response or intolerance to one or more TNF blockers. ‡Upadacitinib is not indicated for MTX-naïve, csDMARD-IR and MTX-IR patients. §Abatacept is indicated for the first-line treatment of moderate to severe rheumatoid arthritis.
SELECT-BEYOND (Bio-IR):* ACR Responses at Week 1 and 122,3
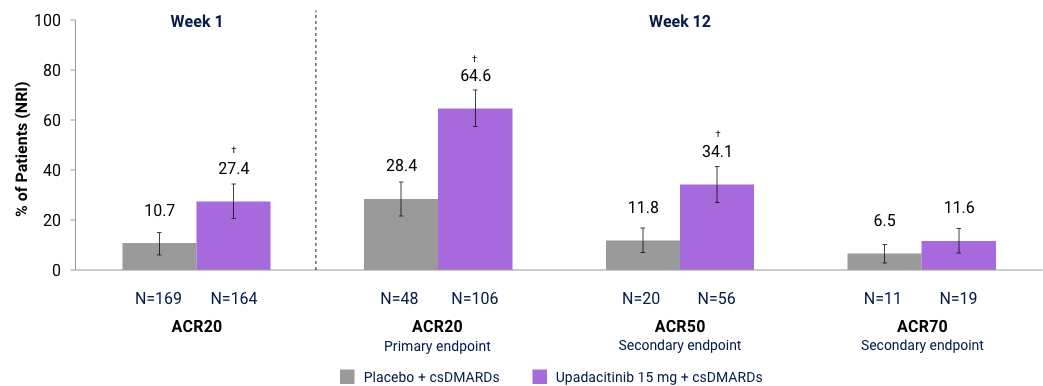
Full analysis set (NRI). *Upadacitinib is only indicated for patients who have had an inadequate response or intolerance to one or more TNF blockers. Comparisons not adjusted for multiplicity: †Nominal p≤0.001 for upadacitinib vs placebo.
SELECT-BEYOND (Bio-IR):* LDA and Clinical Remission† up to Week 2603,23
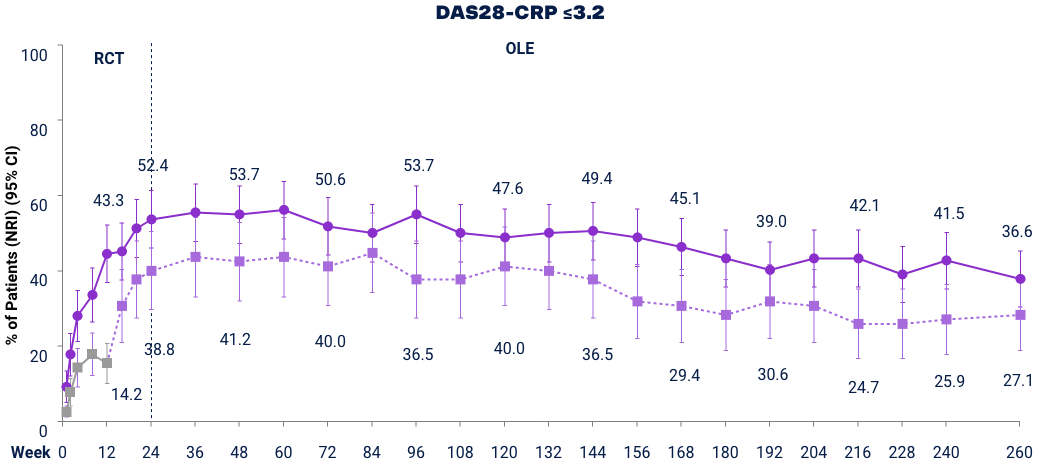
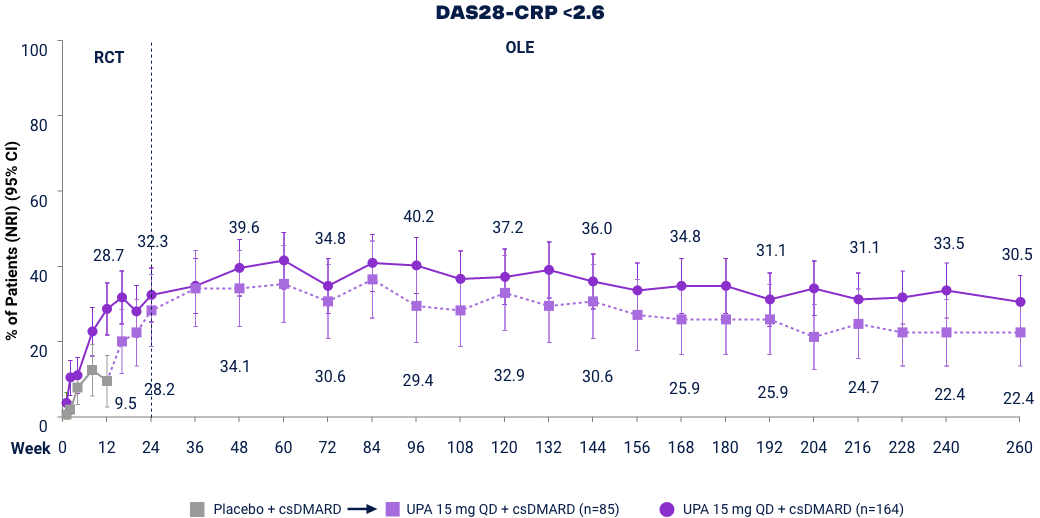
Full analysis set (NRI). DAS28-CRP ≤3.2 was a ranked key secondary endpoint. DAS28-CRP <2.6 was an additional endpoint. At Week 12 patients on PBO were switched to UPA. Placebo includes only those that switched from PBO to UPA 15 mg at Week 12 after original randomization. Does not include those that were originally randomized to PBO and switched to UPA 30 mg. *UPA is only indicated for patients who have had an inadequate response or intolerance to one or more TNF blockers. †Remission does not mean drug-free remission or complete absence of disease activity.
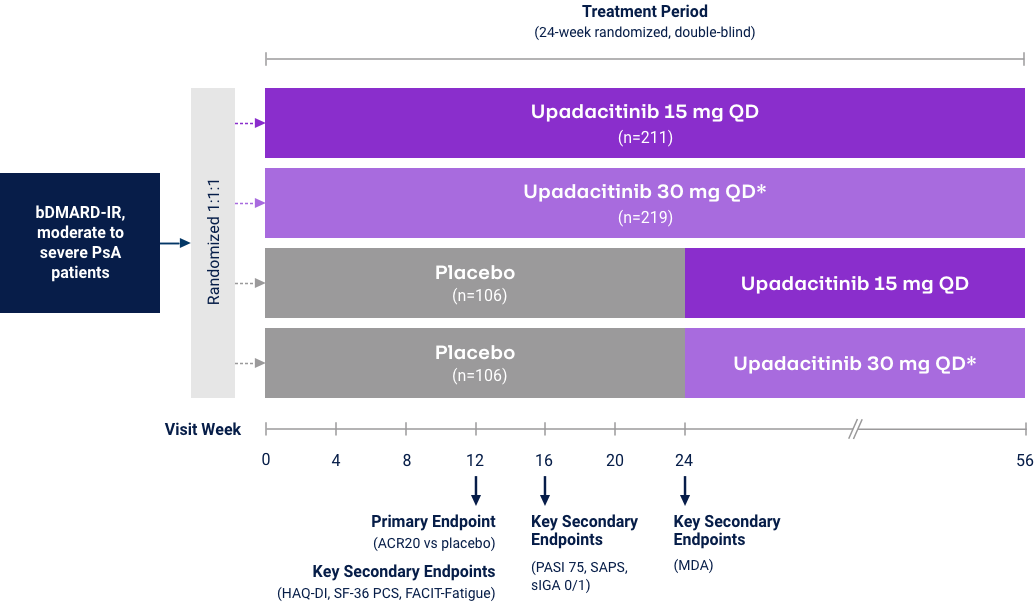
Psoriatic Arthritis
Proportion of Patients Achieving Minimal Disease Activity (MDA)
Differences in study design and populations precludes comparison between individual products and studies.
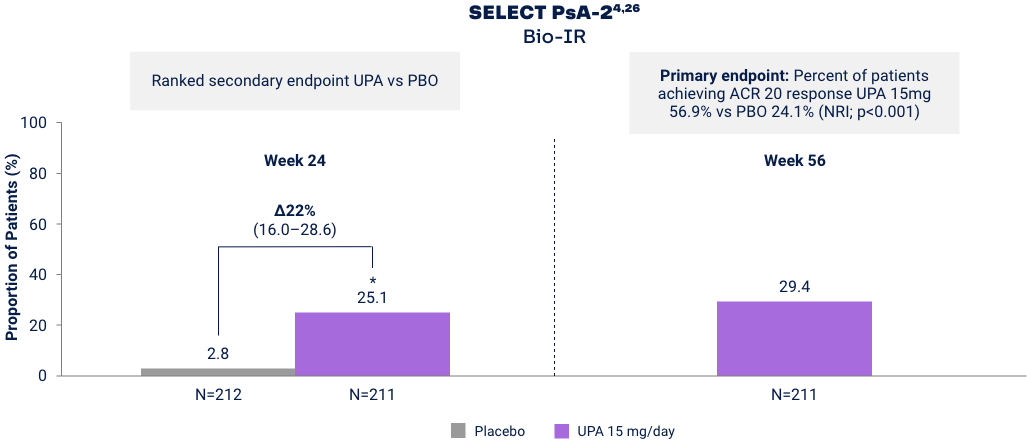
MDA is achieved when meeting 5 of 7 outcome measures27
1. Number of tender joints ≤1
2. Number of swollen joints ≤1
3. PASI ≤1 or BSA-PsO ≤3%
4. Patient assessment of pain ≤1.5 (0–10 NRS)
5. PtGA of disease activity ≤2 (0–10 NRS)
6. Disability Index HAQ-DI ≤0.5
7. Tender entheseal points ≤1
*p<0.001 vs placebo in the multiplicity-controlled analysis. For psoriatic arthritis, upadacitinib is only indicated in patients who have had an inadequate response or intolerance to one or more TNF blockers. Mixed psoriatic arthritis population study includes Bio-IR (47%) and Bio-Naïve (53%) patients.
Adverse Events of Special Interest24
2 Phase 3 Trials
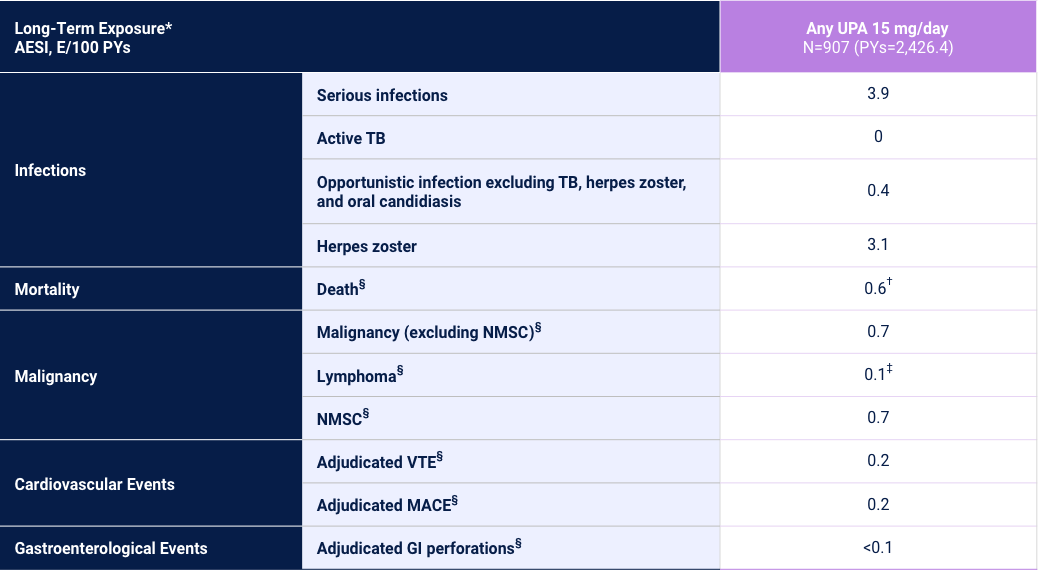
Adverse reaction rates observed in clinical trials and long-term extension studies may not predict rates observed in clinical practice. *Long-Term Exposure: Data as of 6/2021. †Includes treatment-emergent and non-treatment-emergent deaths. ‡All 3 cases Reported as abnormal lymphocyte morphology and not confirmed to be lymphoma. §Data shown are n, n/100 PYs (95% CI).
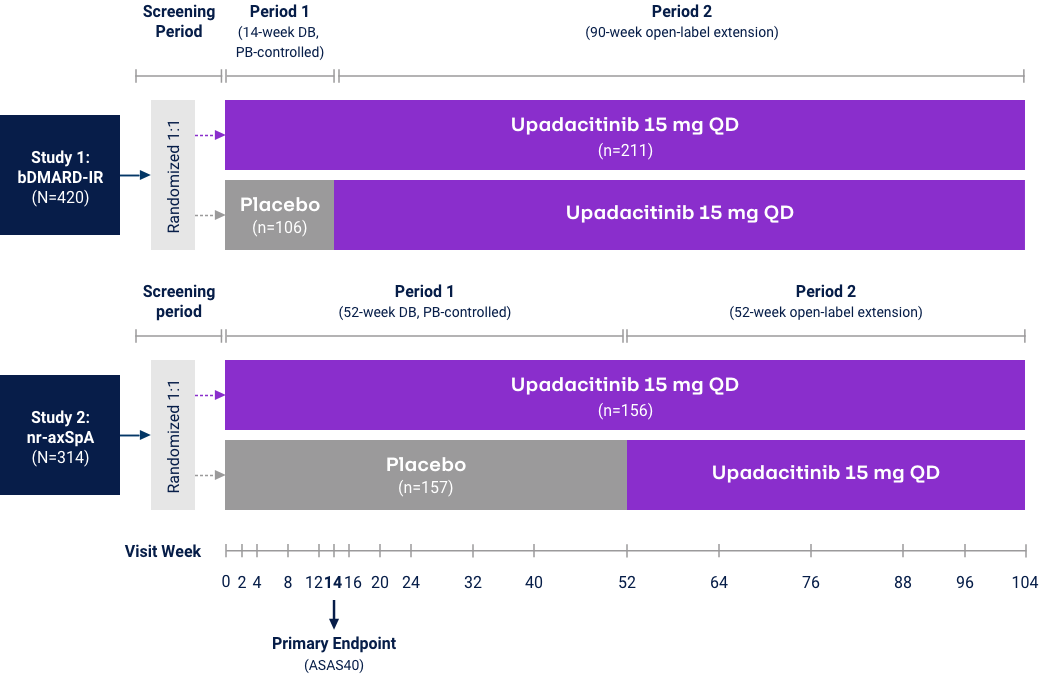
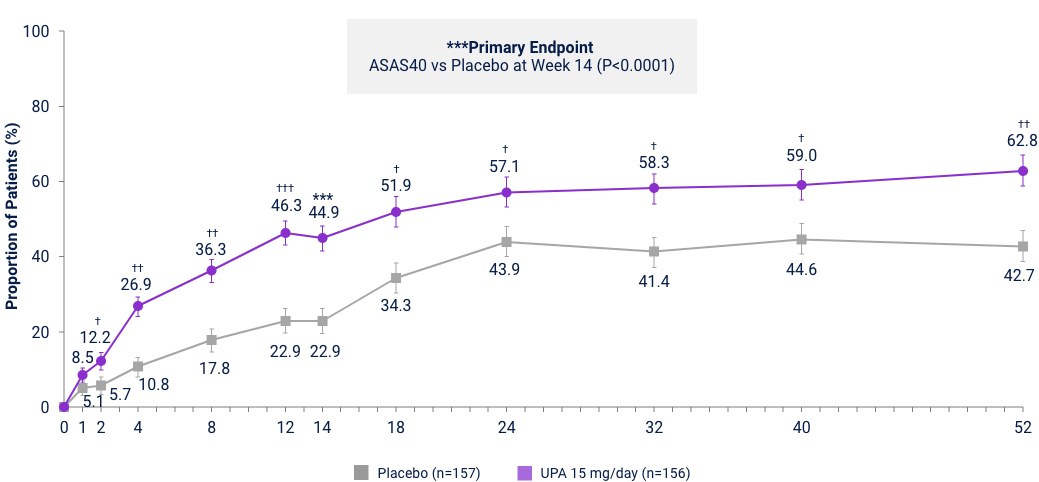
Axial Spondyloarthritis
AS ASAS40 Response Met in nr-AxSpA and
AS bDMARD-IR Patient Populations
nr-axSpA Study6,28
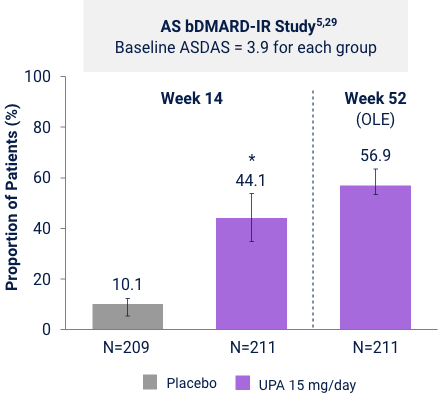
AS bDMARD-IR Study5,29
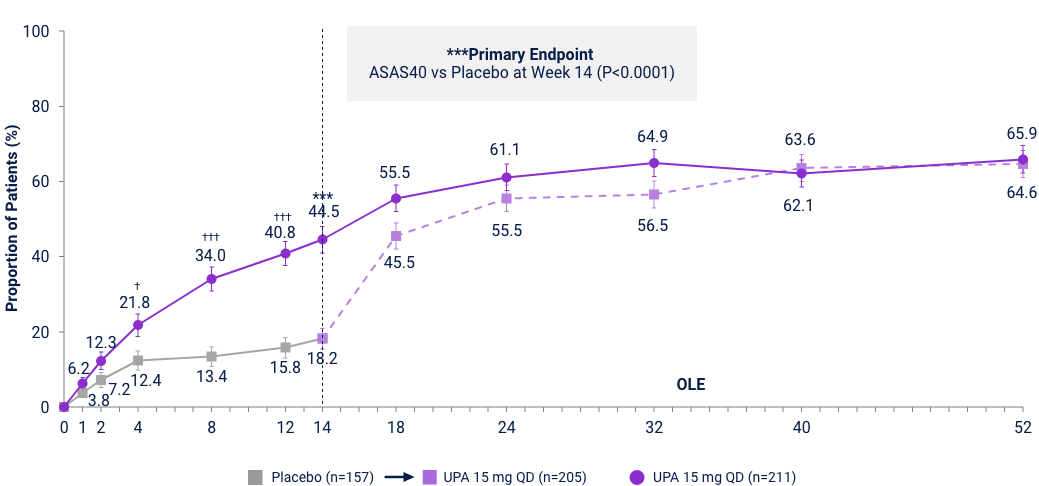
UPA is only indicated for patients with inadequate response or intolerance to a TNF blocker. Limitation: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out. Error bars show 95% CI. Significant in multiplicity-controlled analysis: ***P<0.0001; Without adjustment for multiplicity (nominal): †P<0.05, ††P<0.001, †††P<0.0001.
Proportion of patients Achieving ASDAS LDA in nr-axSpA
and bDMARD-IR AS Studies
Ranked Secondary Endpoint
ASDAS LDA (ASDAS <2.1) vs PBO at Week 14
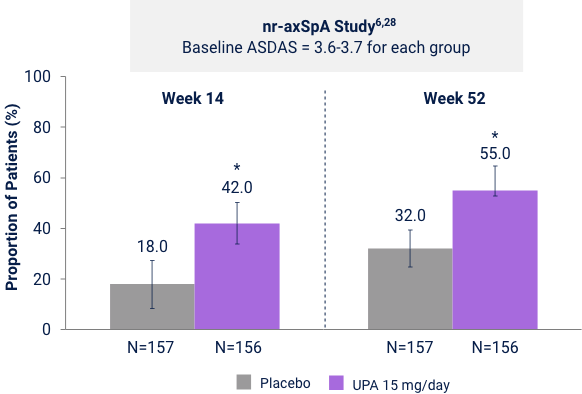
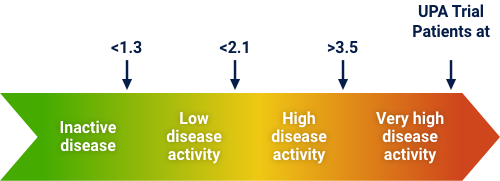
Five items were combined to give a single disease activity score. The ASDAS categorizes disease activity as inactive, low, high, or very high:30
1. Neck, back, or hip pain (BASDAI Q2)
2. Duration of morning stiffness (BASDAI Q6)
3. PtGA: Patient Global Assessment (0-10)
4. Peripheral pain/swelling (BASDAI Q3)
5. CRP (mg/L) or ESR (mm/hr)
*p<0.0001. UPA is only indicated following an inadequate response or intolerance to a TNF blocker. Limitation: In an OLE, there is a potential for enrichment of the
Adverse Events of Special Interest24
1 Phase 2/3 Trial and 1 Phase 3 Trial
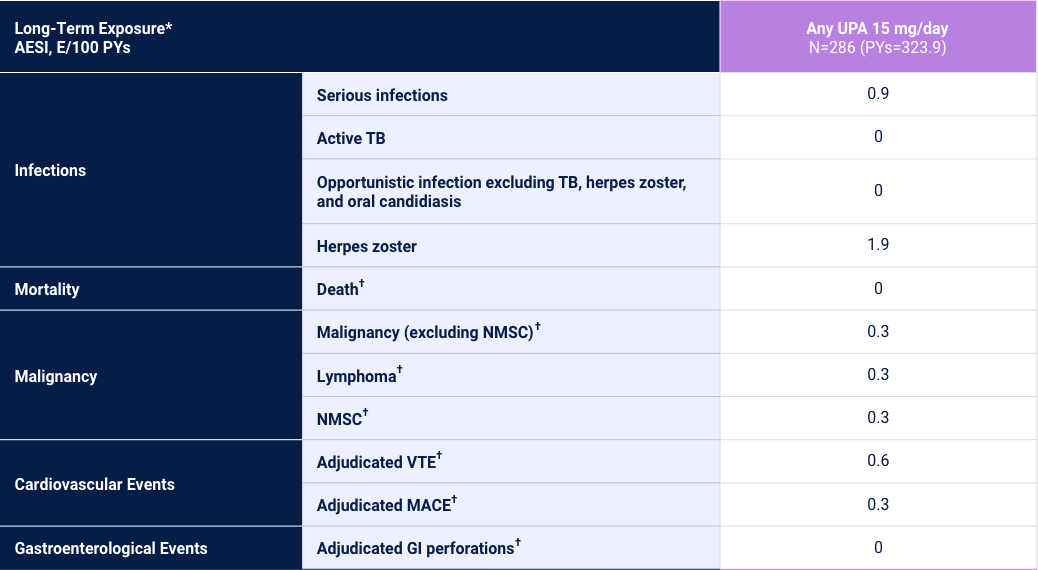
Adverse reaction rates observed in clinical trials and long-term extension studies may not predict rates observed in clinical practice. *Long-Term Exposure: Data as of 6/2021. †Data shown are n, n/100 PYs (95% CI).
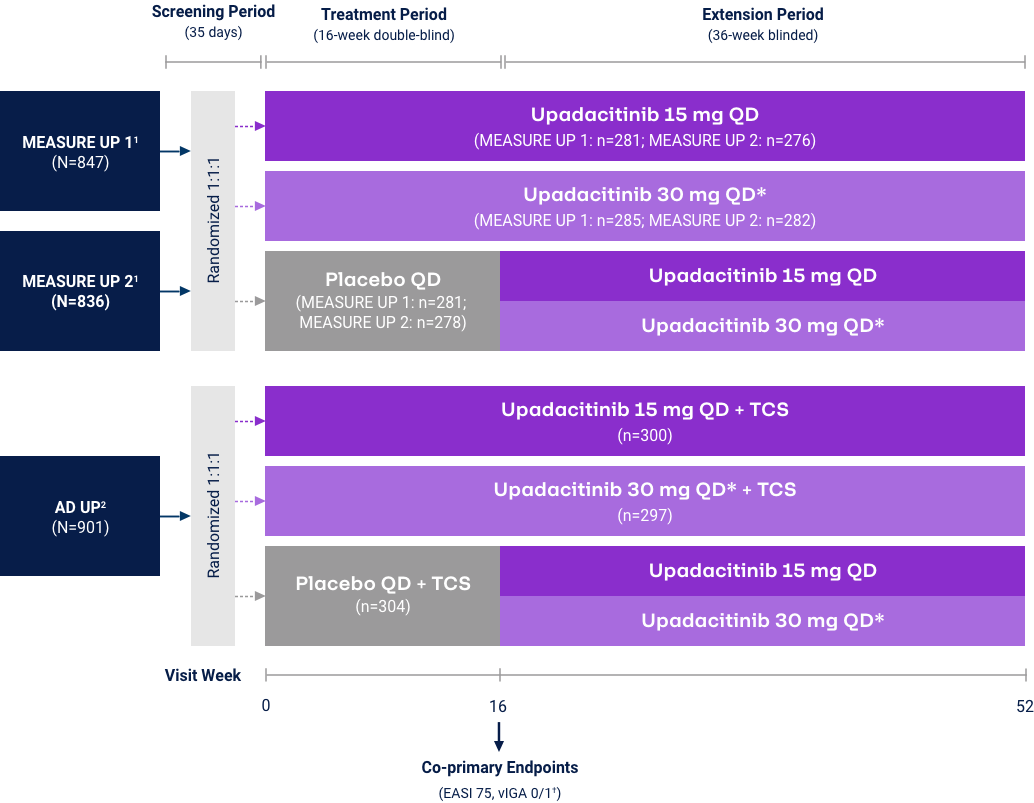
Atopic Dermatitis
v-IGA 0/1 Responses through Week 5231,32
Measure Up 1 & 2
(ITT, NRI-C)
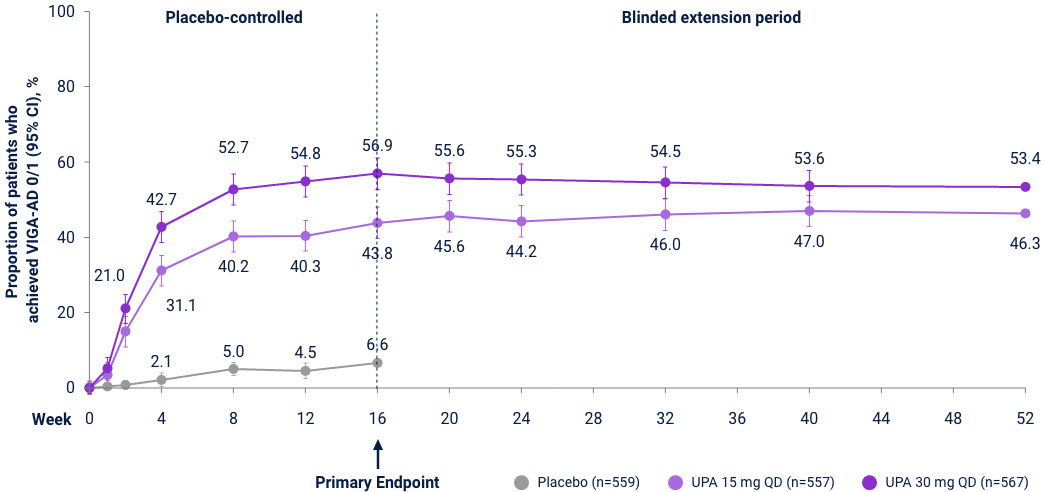
AD Up
(ITT, MI, NRI-C)
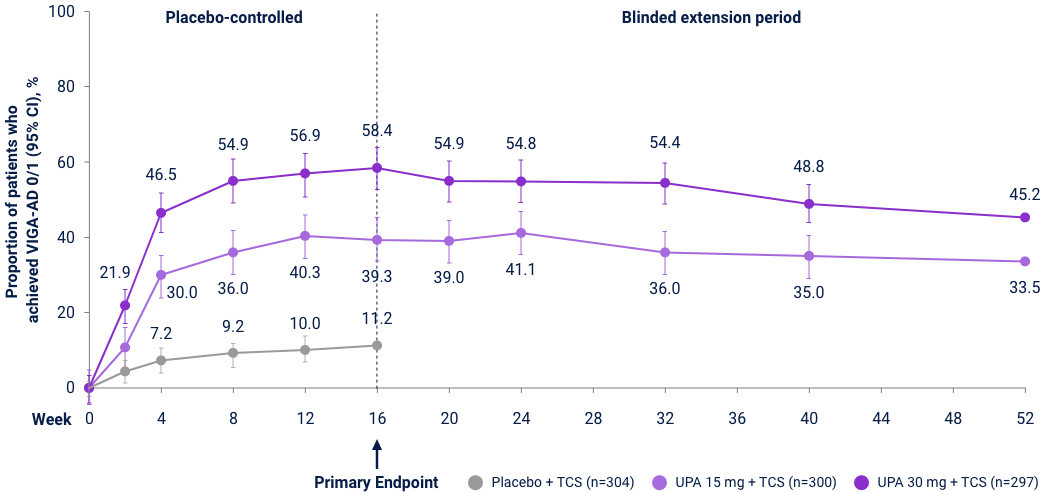
Reserve Upadacitinib 30 mg daily for appropriate patients <65 years old who have an inadequate response to 15 mg. *P<0.01, ***P<0.001 vs PBO+TCS; P-values represented in this graphic are nominal and were not multiplicity controlled. No statistical comparisons were made after Week 16.
Adverse Events of Special Interest33
3 Phase 3 Trials in Adults and Adolescents
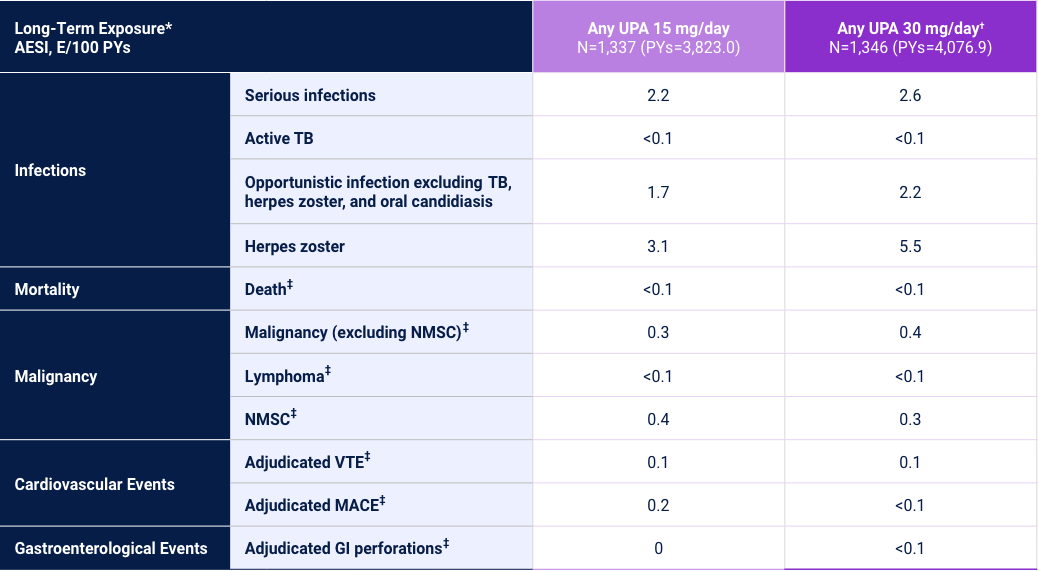
Adverse reaction rates observed in clinical trials and long-term extension studies may not predict rates observed in clinical practice. *Long-Term Exposure: Data as of 8/2022. †UPA 30 mg/day may be considered for appropriate patients <65 years old who have an inadequate response to UPA 15 mg/day. Review the full Prescribing Information for more details. ‡Data shown are n, n/100 PYs (95% CI).
Ulcerative Colitis
Primary Endpoints of Clinical Remission at Weeks 8 and 52
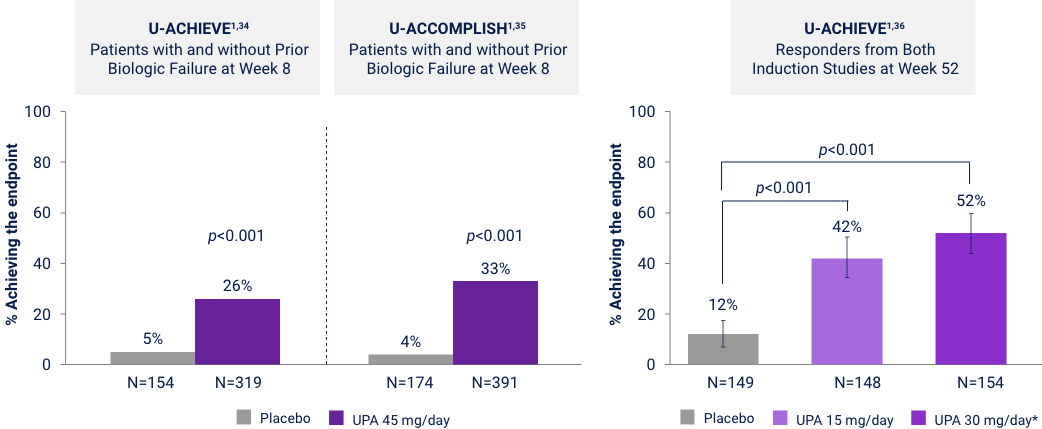
UPA is only indicated for patients with inadequate response or intolerance to a TNF blocker. UPA 30 mg QD may be considered for patients with refractory, severe, or extensive disease. Clinical Remission: Modified Mayo score ≤2, with SFS ≤1 and not greater than baseline, RBS of 0, and endoscopic subscore ≤1 without friability. Error bars represent 95% CI. *UPA 30 mg QD may be considered for patients with refractory, severe, or extensive disease.
Ranked Secondary Endpoints at Week 8
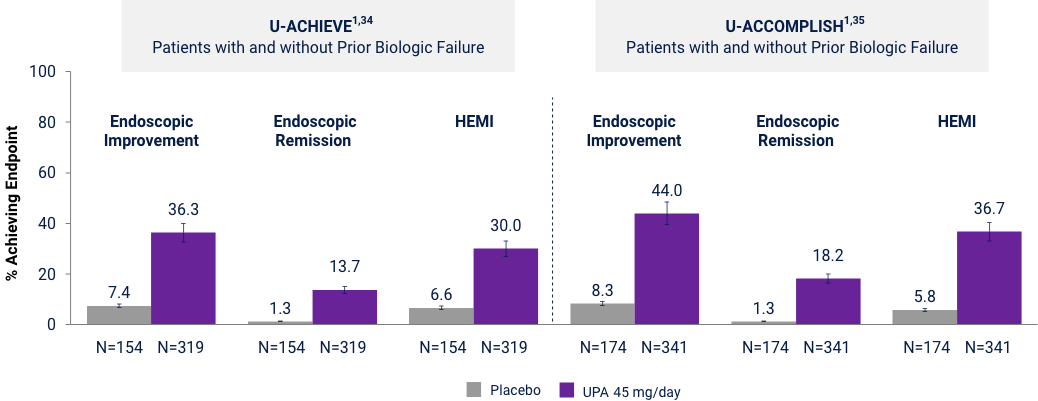
p<0.001. UPA is only indicated for patients with inadequate response or intolerance to a TNF blocker. Endoscopic Improvement: Mayo endoscopic subscore ≤1 without friability. Endoscopic Remission: defined as Mayo endoscopic subscore 0. Histologic Endoscopic Mucosal Improvement (HEMI): Mayo endoscopic subscore of 0 or 1 without friability and Geboes score ≤3.1 indicating neutrophil infiltration <5% of crypts, no crypt destruction and no erosions, ulcerations or granulation tissue. Error bars represent 95% CI.
Adverse Events of Special Interest24
1 Phase 3 Trial
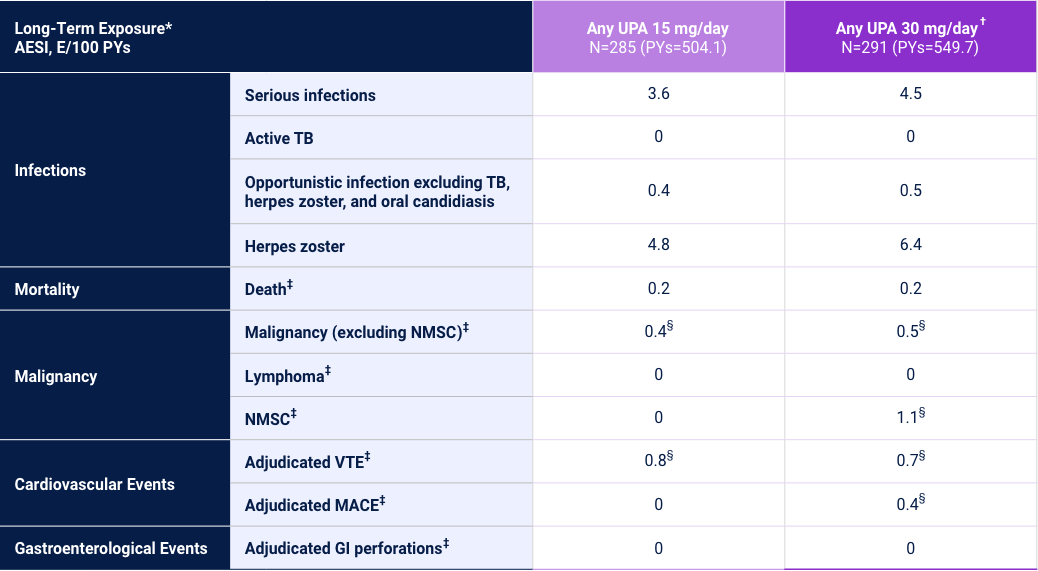
Adverse reaction rates observed in clinical trials and long-term extension studies may not predict rates observed in clinical practice. *Long-Term Exposure: Data as of 4/2021. †UPA 30 mg/day may be considered for patients with refractory, severe, or extensive disease. Review the full Prescribing Information for more details. ‡Data shown are n, n/100 PYs (95% CI). §Data shown are E/100 PYs.
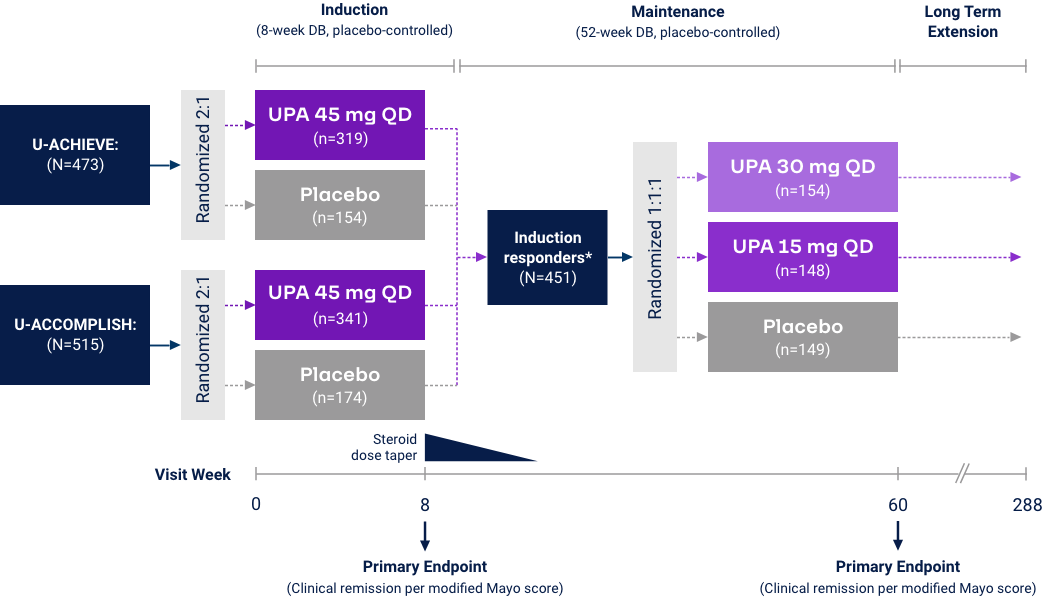
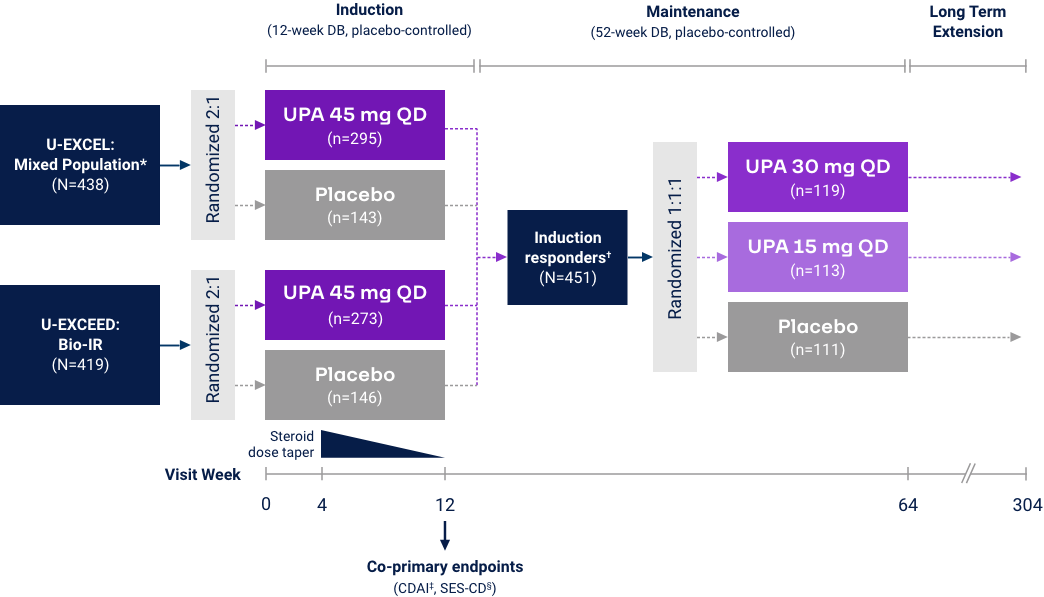
Crohn’s Disease
Clinical Remission (CDAI < 150) at Weeks 12 and 521*
Co-Primary Endpoint
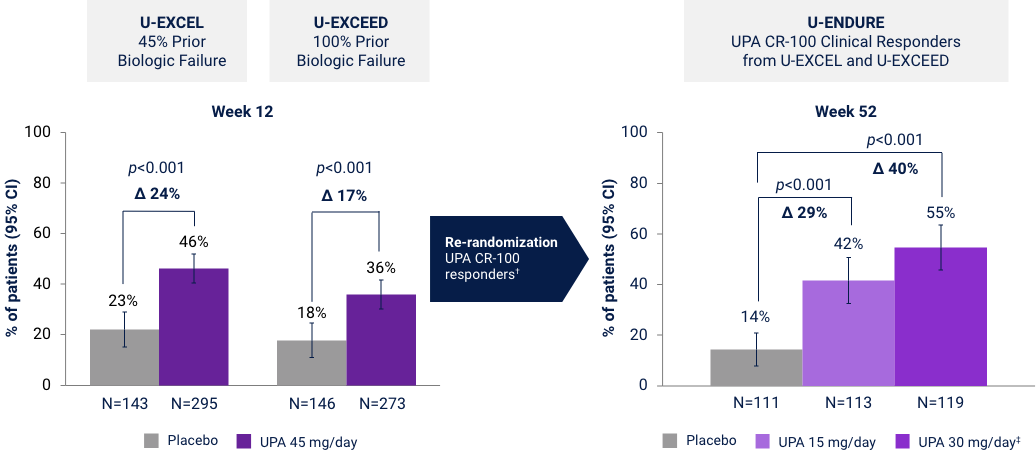
*UPA is only indicated for patients with inadequate response or intolerance to a TNF blocker. †Efficacy data in U-ENDURE are based on patients who achieved CR-100 clinical response at Week 12 of induction. ‡UPA 30 mg QD may be considered for patients with refractory, severe, or extensive disease. CR-100: decrease of ≥100 points in CDAI from baseline. Clinical Remission (CDAI):
Endoscopic Response at Weeks 12 and 521*
Co-Primary Endpoint
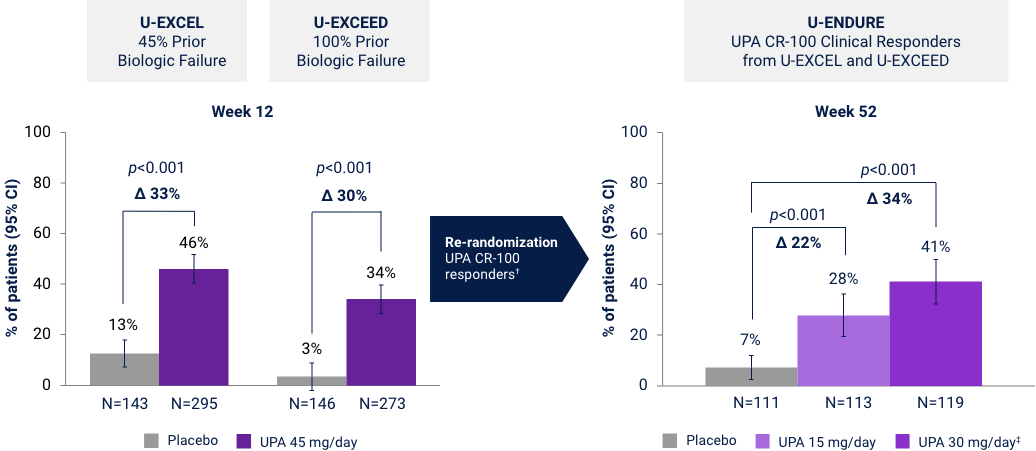
*UPA is only indicated for patients with inadequate response or intolerance to a TNF blocker. †Efficacy data in U-ENDURE are based on patients who achieved CR-100 clinical response at Week 12 of induction. ‡UPA 30 mg QD may be considered for patients with refractory, severe, or extensive disease. CR-100: decrease of ≥100 points in CDAI from baseline. Endoscopic Response: Decrease in SES-CD >50% from baseline (or for subjects with isolated ileal disease and a baseline SES-CD of 4, at least a 2-point reduction from baseline), scored by central reader.
Endoscopic Remission at Weeks 12 and 52
Prior Biologic Failure Subgroup Analysis37*†
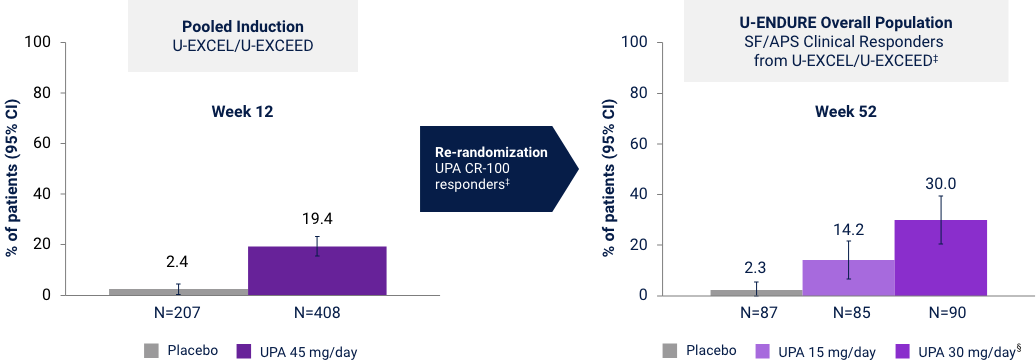
Endoscopic remission: SES-CD ≤4 and no subscore >1 in any individual variable, as scored by central reviewer. *UPA is only indicated for patients with inadequate response or intolerance to a TNF blocker. †No statistical inferences of the prior biologic failure data can be made. ‡Efficacy data in U-ENDURE are based on patients who achieved CR-100 clinical response (decrease of ≥100 points in CDAI from baseline) at Week 12 of induction. §UPA 30 mg QD may be considered for patients with refractory, severe, or extensive disease.
Corticosteroid-free Clinical Remission (CDAI < 150)
in Patients with Corticosteroid Use at Baseline
Prior Biologic Failure Subgroup Analysis37*†
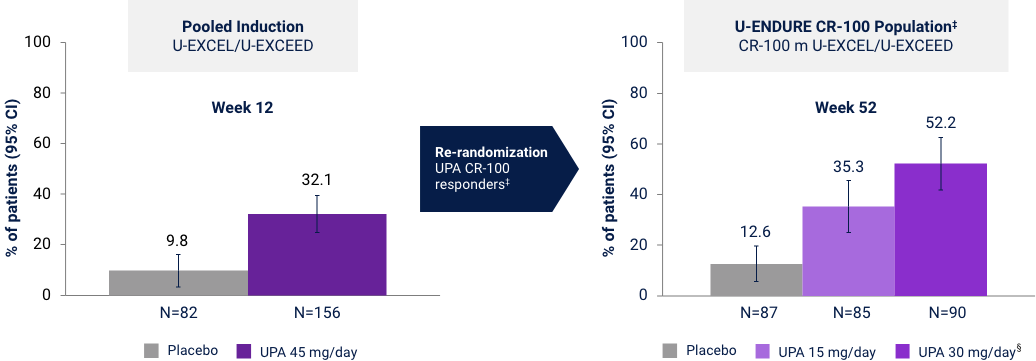
Induction: Discontinuation of corticosteroids AND achievement of clinical remission per CDAI (CDAI <150) among patients on corticosteroids at baseline. Maintenance: Discontinuation of corticosteroids for at least 90 days prior to Week 52 AND achievement of clinical remission per CDAI (CDAI <150) among patients on corticosteroids at baseline. *UPA is only indicated for patients with inadequate response or intolerance to a TNF blocker. †No statistical inferences of prior biologic failure data can be made. ‡Efficacy data in U-ENDURE are based on patients who achieved CR-100 clinical response (decrease of ≥100 points in CDAI from baseline) at Week 12 of induction. §UPA 30 mg QD may be considered for patients with refractory, severe, or extensive disease.
Cross-indication Long-term Safety Data for Upadacitinib
(AD, RA, PsA, AS, nr-axSpA, UC, and CD)
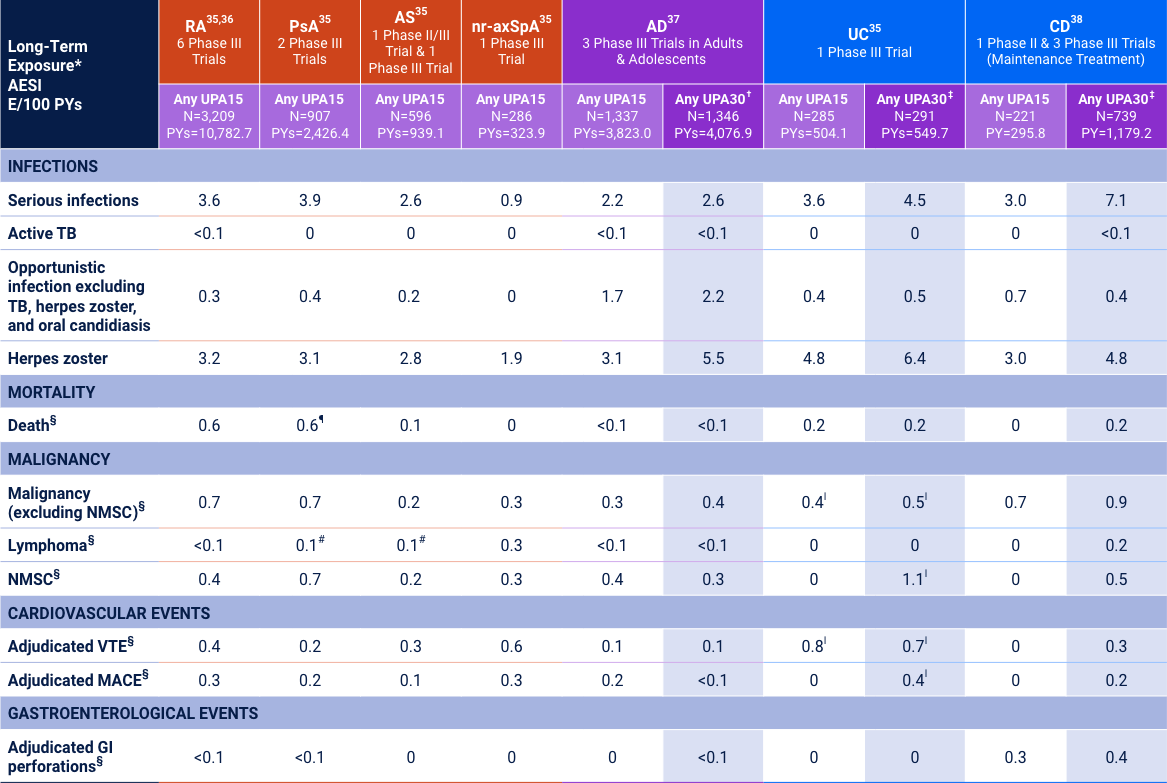
Adverse reaction rates observed in clinical trials and long-term extension studies may not predict rates observed in clinical practice. *Long-Term Exposure: Data as of 6/2021 except AD as of 8/2022, UC as of 4/2021, and CD as of 2/2023. †UPA30 may be considered for appropriate patients <65 years old who have an inadequate response to UPA15. Review the full Prescribing Information for more details. ‡UPA30 mg may be considered for patients with refractory, severe, or extensive disease. §Data shown are n, n/100 PYs (95% CI). ¶Includes treatment-emergent and non–treatment-emergent deaths. ‖Data shown are E/100 PYs. #All 3 cases Reported as abnormal lymphocyte morphology and not confirmed to be lymphoma.
Upadacitinib Indications, Important Safety Considerations, and Boxed Warning
INDICATIONS
Upadacitinib is a Janus kinase (JAK) inhibitor indicated for the treatment of:
- Adults with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to one or more tumor necrosis factor (TNF) blockers.
- Adults with active psoriatic arthritis (PsA) who have had an inadequate response or intolerance to one or more TNF blockers.
- Adults with active ankylosing spondylitis (AS) who have had an inadequate response or intolerance to one or more TNF blockers.
- Adults with active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation who have had an inadequate response or intolerance to TNF blocker therapy.
- Limitations of Use for RA, PsA, AS and nr-axSpA: Upadacitinib is not recommended for use in combination with other JAK inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
- Adults and pediatric patients 12 years of age and older with refractory moderate to severe atopic dermatitis (AD) whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.
- Limitations of Use for AD: Upadacitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
- Adults with moderately to severely active ulcerative colitis (UC) who have had an inadequate response or intolerance to one or more TNF blockers.
- Adults with moderately to severely active Crohn’s disease (CD) who have had an inadequate response or intolerance to one or more TNF blockers.
- Limitations of Use for UC and CD: Upadacitinib is not recommended for use in combination with other JAK inhibitors, biological therapies for UC or CD, respectively, or with potent immunosuppressants such as azathioprine and cyclosporine.
IMPORTANT SAFETY CONSIDERATIONS AND BOXED WARNING
Serious Infections: Patients treated with upadacitinib are at increased risk for developing serious infections that may lead to hospitalization or death. These infections include tuberculosis (TB), invasive fungal, bacterial, viral, and other infections due to opportunistic pathogens. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids. Test for latent TB before and during therapy; treat latent TB prior to use. Consider the risks and benefits prior to initiating therapy in patients with chronic or recurrent infection. If a serious infection develops, interrupt upadacitinib until the infection is controlled.
Mortality: In a postmarketing safety study in RA patients ≥ 50 years of age with at least one cardiovascular (CV) risk factor comparing another JAK inhibitor to TNF blockers, a higher rate of all-cause mortality, including sudden CV death, was observed with the JAK inhibitor.
Malignancies: Malignancies have been observed in upadacitinib treated patients. In RA patients treated with another JAK inhibitor, a higher rate of lymphomas and lung cancers was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with upadacitinib, particularly in patients with a known malignancy (other than a successfully treated non-melanoma skin cancer [NMSC]), patients who develop a malignancy when on treatment, and patients who are current or past smokers. NMSCs have been reported in patients treated with upadacitinib. Periodic skin examination is recommended for patients who are at increased risk for skin cancer. Advise patients to limit sunlight exposure by wearing protective clothing and using sunscreen.
Major Adverse Cardiovascular Events (MACE): In RA patients who were ≥ 50 years of age with at least one CV risk factor treated with another JAK inhibitor, a higher rate of MACE (CV death, myocardial infarction, and stroke) was observed compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with upadacitinib. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur. Discontinue upadacitinib in patients that have experienced a myocardial infarction or stroke.
Thrombosis: Thrombosis, including deep vein thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated with JAK inhibitors, including upadacitinib. Many of these adverse events were serious and some resulted in death. In RA patients who were ≥ 50 years of age with at least one CV risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid upadacitinib in patients at risk. Patients with symptoms of thrombosis should discontinue upadacitinib and be promptly evaluated.
Hypersensitivity Reactions: Upadacitinib is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients. Serious hypersensitivity reactions such as anaphylaxis and angioedema were reported in patients receiving upadacitinib in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue upadacitinib and institute appropriate therapy.
Other Serious Adverse Reactions: Patients treated with upadacitinib also may be at risk for other serious adverse reactions, including gastrointestinal perforations, neutropenia, lymphopenia, anemia, lipid elevations, liver enzyme elevations, and embryo-fetal toxicity. If upadacitinib exposure occurs during pregnancy, please report the pregnancy to the surveillance program by calling 1-800-633-9110.
Vaccinations: Avoid use of live vaccines during, or immediately prior to, upadacitinib therapy. Prior to initiating upadacitinib, it is recommended that patients be brought up to date with all immunizations, including prophylactic varicella zoster or herpes zoster vaccinations, in agreement with current immunization guidelines.
Medication Residue in Stool: Reports of medication residue in stool or ostomy output have occurred in patients taking upadacitinib. Most reports described patients with shortened gastrointestinal transit times. Instruct patients to contact their healthcare provider if medication residue is observed repeatedly.
Common Adverse Reactions in RA, PsA, AS and nr-axSpA: The most common adverse reactions (≥1%) were upper respiratory tract infections, herpes zoster, herpes simplex, bronchitis, nausea, cough, pyrexia, acne, and headache.
Common Adverse Reactions in AD: The most common adverse reactions (≥1%) are upper respiratory tract infections, acne, herpes simplex, headache, increased blood creatine phosphokinase, cough, hypersensitivity, folliculitis, nausea, abdominal pain, pyrexia, increased weight, herpes zoster, influenza, fatigue, neutropenia, myalgia, and influenza like illness.
Common Adverse Reactions in UC: The most common adverse reactions (≥5%) reported are upper respiratory tract infections, increased blood creatine phosphokinase, acne, neutropenia, elevated liver enzymes, and rash.
Common Adverse Reactions in CD: The most common adverse reactions (≥5%) reported are upper respiratory tract infections, anemia, pyrexia, acne, herpes zoster, and headache.
Review upadacitinib full Prescribing Information for additional information, visit www.rxabbvie.com or contact AbbVie Medical Information at 1-800-633-9110.
ABAT=abatacept; ACR=American College of Rheumatology; ACR20/50=20%/50% improvement in American College of Rheumatology score; AESI=adverse event of special interest; ASAS40=40% improvement in the Assessment of SpondyloArthritis International Society score; ADA=adalimumab; ASDAS=Ankylosing Spondylitis Disease Activity Score; Bio=biologic; CDAI=Clinical Disease Activity Index; CI=confidence interval; cMTX=continuous methotrexate; CR=clinical remission; csDMARD=conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP=28-joint Disease Activity Score based on C-reactive protein; DUP=dupilumab; EASI75/100=75%/100% improvement on the EASI score; FACIT-F=Functional Assessment of Chronic Illness Therapy-Fatigue; GI=gastrointestinal; HAQ-DI=Health Assessment Questionnaire–Disability Index; IR=inadequate response; ITT= intent to treat; LDA=low disease activity; MACE=major adverse cardiovascular event; MDA=minimal disease activity; mTSS=modified total Sharp/van der Heijde score; MTX=methotrexate; N/A=measure was not a primary or ranked secondary endpoint for the trial; NMSC=nonmelanoma skin cancer; NRI=non-responder imputation; NRI-C=non-responder imputation with multiple imputation to handle missing data due to COVID-19; OLE=open-label extension; PBO=placebo; PCS=physical component summary; PsA=psoriatic arthritis; PsO=psoriasis; PY=patient-year; QD=once daily; RA=rheumatoid arthritis; RBS=rectal bleeding subscore; RCT=randomized controlled trial; SEC=secukinumab; SES-CD=simple endoscopic activity score for Crohn’s disease; SF-36=36-Item Short Form Survey; SFS=stool frequency subscore; TB=tuberculosis; TEAE=treatment-emergent adverse event; TNF=tumor necrosis factor; UPA=upadacitinib; UST=ustekinumab; vIGA-AD 0/1, validated Investigator Global Assessment for atopic dermatitis of clear (0) or almost clear (1) with ≥2 grades of improvement; VTE=venous thromboembolism.
References: 1. RINVOQ (upadacitinib) [package insert]. North Chicago, IL: AbbVie, Inc. 2022. 2. Genovese MC et al. Lancet. 2018;391(10139):2513-2524. 3. Data on file, AbbVie Inc. ABVRRTI72945. 4. Mease PJ et al. Ann Rheum Dis. 2021;80(3):312-320. 5. van der Heijde D et al. Ann Rheum Dis. 2022;81(11):1515-1523. 6. Deodhar A et al. Lancet. 2022;400(10349):369-379. 7. Guttman-Yassky E et al. Lancet. 2021;397(10290):2151-2168. 8. Loftus EV et al. Oral Presentation at Digestive Disease Week 2022. 9. Colombel J-F et al. Oral Presentation at DDW 2022. 10. Panes J et al. Oral Presentation at ACG 2022. 11. Vermeire S et al. UEGW Virtual Congress 2021. #OP017. 12. Fleischmann R et al. EULAR e-congress 2021. Poster POS0087. 13. Simpson et al. Poster presented DERM 2021. 14. ClinicalTrials.Gov. NCT03345823. 15. Rubbert-Roth A et al. N Engl J Med. 2020;383(16):1511-1521 and Supplement. 16. McInnes IB et al. N Engl J Med. 2021;384(13):1227-1239. 17. Blauvelt A et al. JAMA Dermatol. 2021;157(9):1047-1055. 18. Fleischmann R et al. Arthritis Rheumatol. 2019;71(11):1788-1800. 19. Burmester GR et al. Lancet. 2018;391(10139):2503-2512. 20. Smolen JS et al. Lancet. 2019;393(10188):2303-2311. 21. van Vollenhoven R et al. Arthritis Rheumatol. 2020;72(10):1607-1620. 22. Data on file, AbbVie Inc. ABVRRTI68885. 23. Data on file, AbbVie Inc. ABVRRTI74946. 24. Data on file, AbbVie Inc. ABVRRTI74922. 25. Data on file, AbbVie Inc. ABVRRTI75356. 26. Mease PJ et al. Rheumatol Ther. 2021;8:903-919. 27. Coates LC et al. Ann Rheum Dis. 2010;69:48-53. 28. Data on file, AbbVie Inc. ABVRRTI74952. 29. Data on file, AbbVie Inc. ABVRRTI74951. 30. ASDAS calculator. Assessment of SpondyloArthritis international Society (ASAS) website. https://www.asas-group.org/clinical-instruments/asdas-calculator/ 31. Data on file, AbbVie Inc. ABVRRTI72724. 32. Silverberg et al. J Allergy Clin Immunol. 2022;149(3):977-987. 33. Data on file, AbbVie Inc. ABVRRTI77022. 34. Danese S, et al. ECCO Virtual Congress 2021. #OP24. 35. Vermeire S et al. ECCO Virtual Congress 2021. #OP23. 36. Press Release: https://news.abbvie.com/news/press-releases/upadacitinib-rinvoq-met-primary-and-all-secondary-endpoints-in-52-week-phase-3-maintenance-study-in-ulcerative-colitis-patients.htm 37. Data on file, AbbVie Inc. ABVRRTI75991. 38. Data on file, AbbVie Inc. ABVRRTI75992.