SKYRIZI® (risankizumab-rzaa) Overview
*This information relates to an indication that has not been approved by a regulatory agency and is being provided to assist in planning and budgeting for future coverage and/or reimbursement decisions before FDA approval. The safety and efficacy of the product for this indication has not been established. Ulcerative Colitis is in phase III clinical development. There is no guarantee that risankizumab will be approved by the FDA or other global regulatory health authority for this investigational use.
Clinical Remission does not imply drug free remission. Primary and ranked secondary endpoint versus placebo: ¶p<0.05; †p<0.001; §nominal p<0.05 vs placebo. ‡First time when primary endpoint measure separated from placebo. #Ranked secondary endpoints, and were measured at the same time as the primary endpoint.
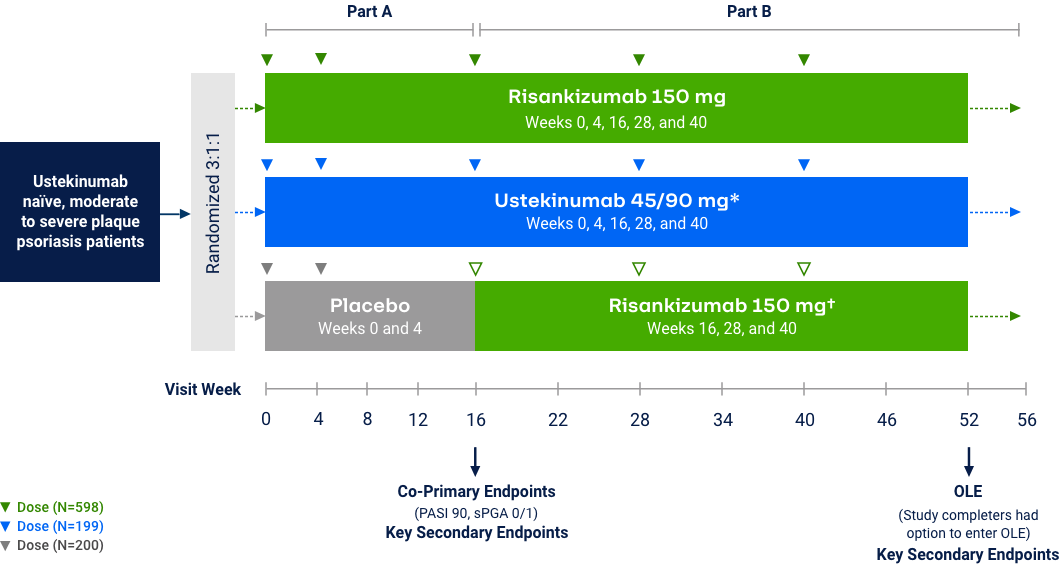
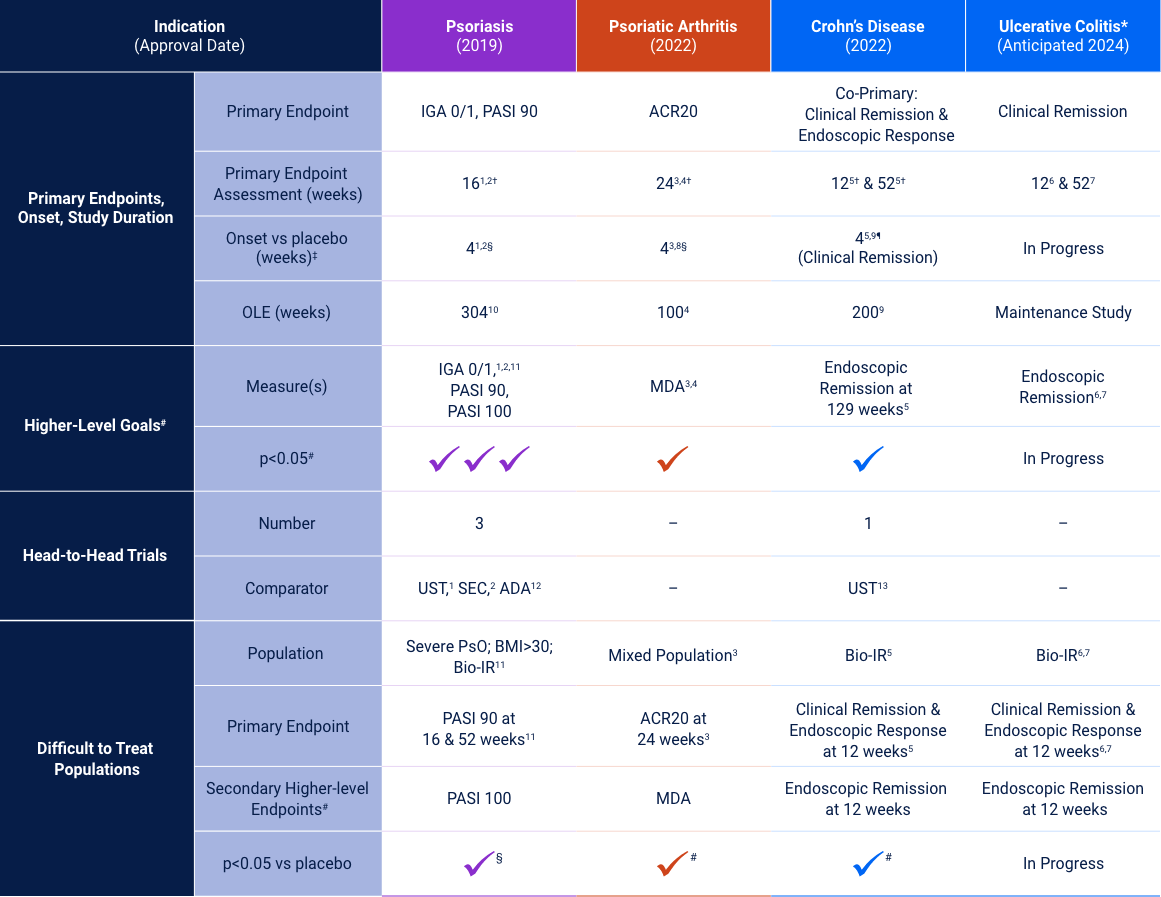
Psoriasis
Risankizumab Phase 3 Program: Efficacy Data at Week 16
The chart is not intended to compare across trial endpoints as patient populations may vary.
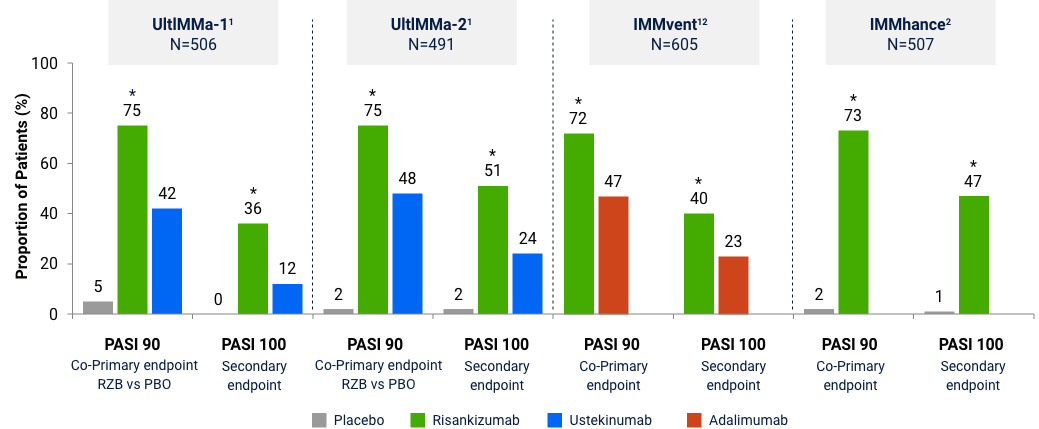
sPGA 0/1 (co-primary endpoint at Week 16)
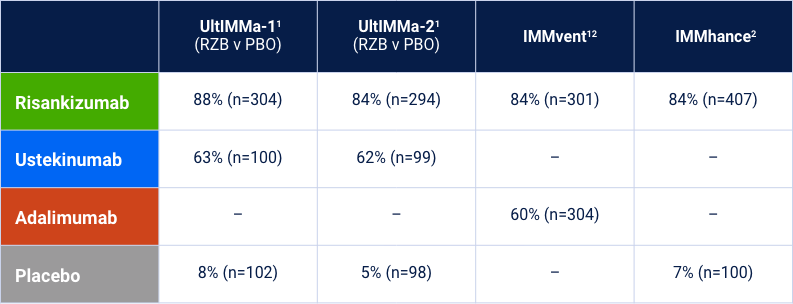
Note: In UltIMMa-1 and -2, the primary end point was Risankizumab vs. Placebo *P<0.001. The comparator (ustekinumab) used in these studies was sourced from the European Union. Comparability to United States licensed ustekinumab has not been demonstrated.
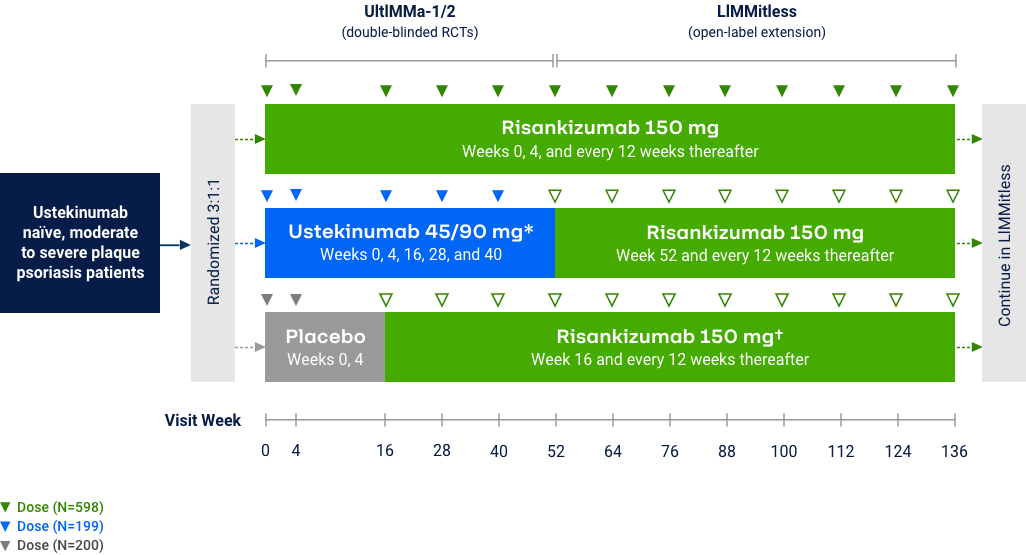
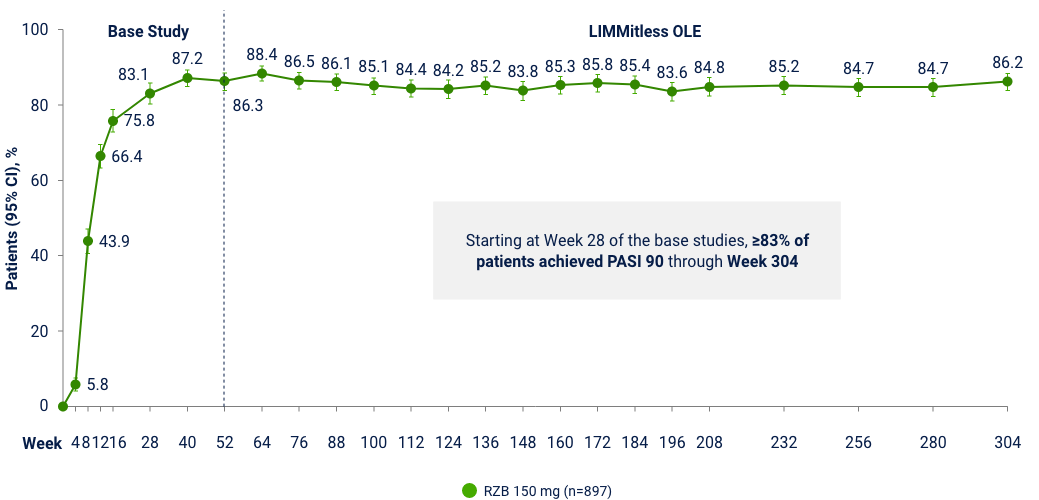
LiMMitless: Long Term PASI 90 Responses at Week 304
Multiple studies, integrated analyses (mNRI)10*
LiMMitless: Long Term PASI 100 Responses at Week 304
Multiple studies, integrated analyses (mNRI)10*
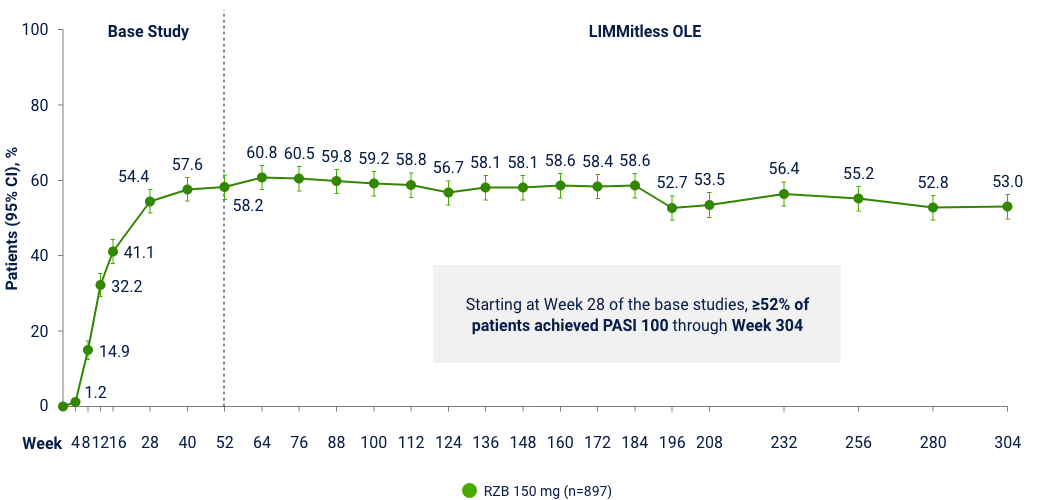
OLE Limitation: In an OLE, there is a potential for enrichment of the long-term data in the remaining patient populations since patients who are unable to tolerate or do not respond to the drug often drop out. *Patients enrolled in base study (UltIMMa-1 and -2, SustaIMM, IMMvent, and NCT03255382) are eligible to enroll into LIMMitless, an open-label extension study. Because of differences in base study lengths, some patients enrolled in LIMMitless earlier than 52 weeks. 706 ongoing patients completed the assessment visit at the data cutoff. In the mNRI analysis, nonresponse is imputed only for treatment failures, defined as patients who have worsening of psoriasis; mixed model is used to estimate parameter value.
Risankizumab Safety in Psoriasis: Events of Interest14-16
Integrated analyses across 5 trials (Week 16) or 20 trials (long-term)
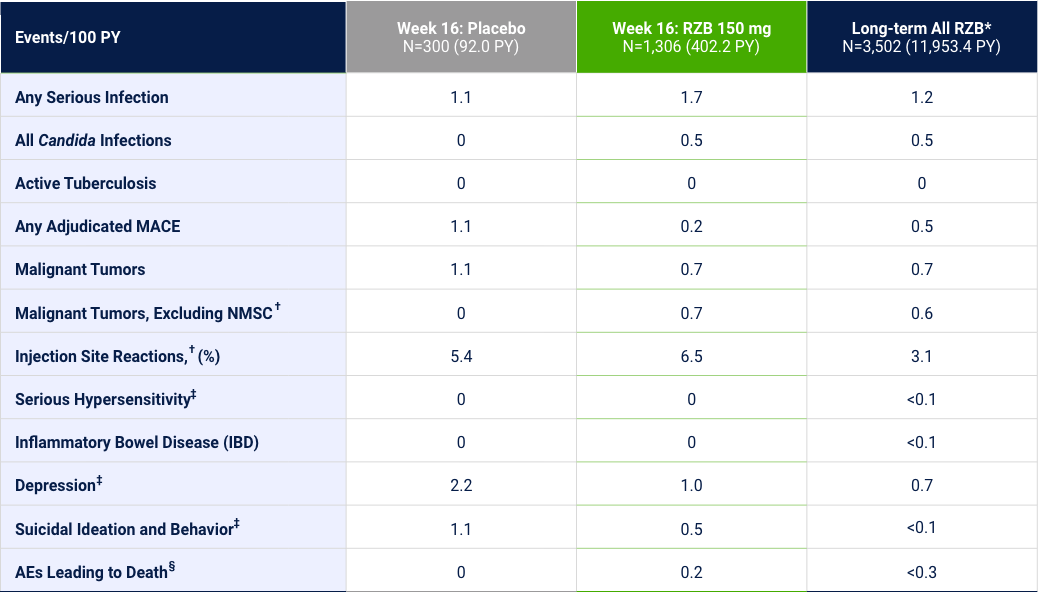
Events counted in the Week 16 column are also included in the Long-term column. *Includes all patients dosed with RZB in phase 1, 2 and 3, including 18 mg, 90 mg, 150 mg, and 180 mg doses. †Identified by company MedDRA query. ‡Identified by standard MedDRA query. §Includes non–treatment-emergent deaths.
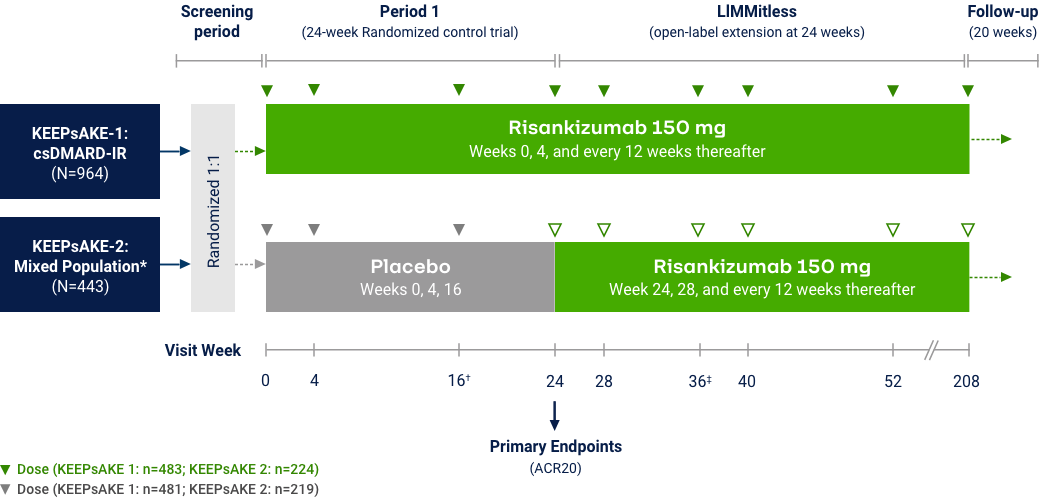
Psoriatic Arthritis
KEEPsAKE-1 and KEEPsAKE-2: Efficacy Endpoints
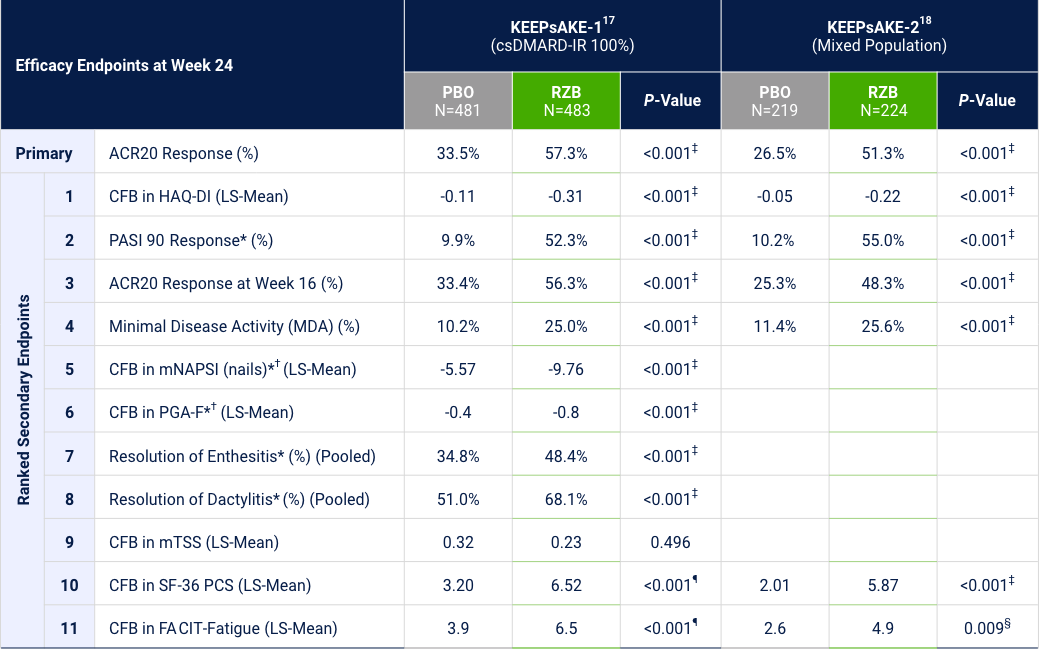
*Among patients with symptom present at baseline. †Risankizumab is not approved to treat nail psoriasis. ‡Statistically significant at 0.001 level. §Statistically significant at <0.01 level. ¶The nominal P-values are provided.
Proportion of Patients Achieving Minimal Disease Activity
Differences in study design and populations precludes comparison between individual products and studies.
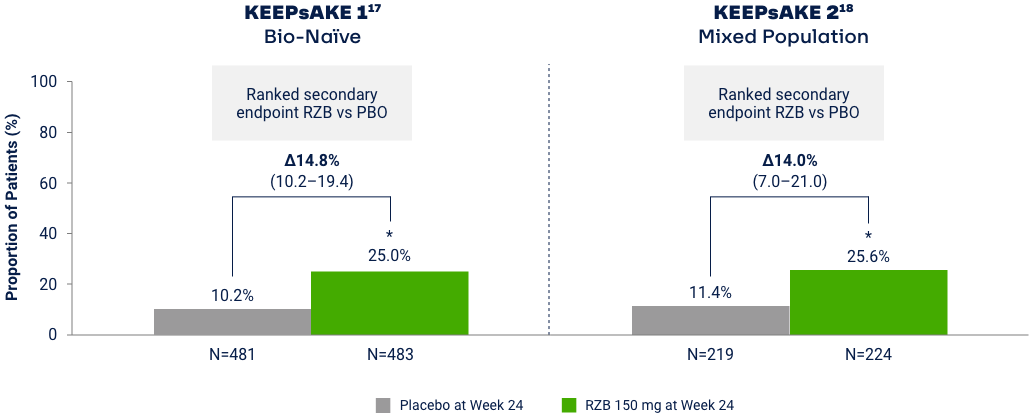
MDA is achieved when meeting 5 of 7 outcome measures19
1. Number of tender joints ≤1
2. Number of swollen joints ≤1
3. PASI ≤1 or BSA-PsO ≤3%
4. Patient assessment of pain ≤1.5 (0–10 NRS)
5. PtGA of disease activity ≤2 (0–10 NRS)
6. Disability Index HAQ-DI ≤0.5
7. Tender entheseal points ≤1
*p<0.001 vs placebo in the multiplicity-controlled analysis.
KEEPsAKE 1 and KEEPsAKE 23,4,20-24
Overview of TEAEs of Interest Through Week 100
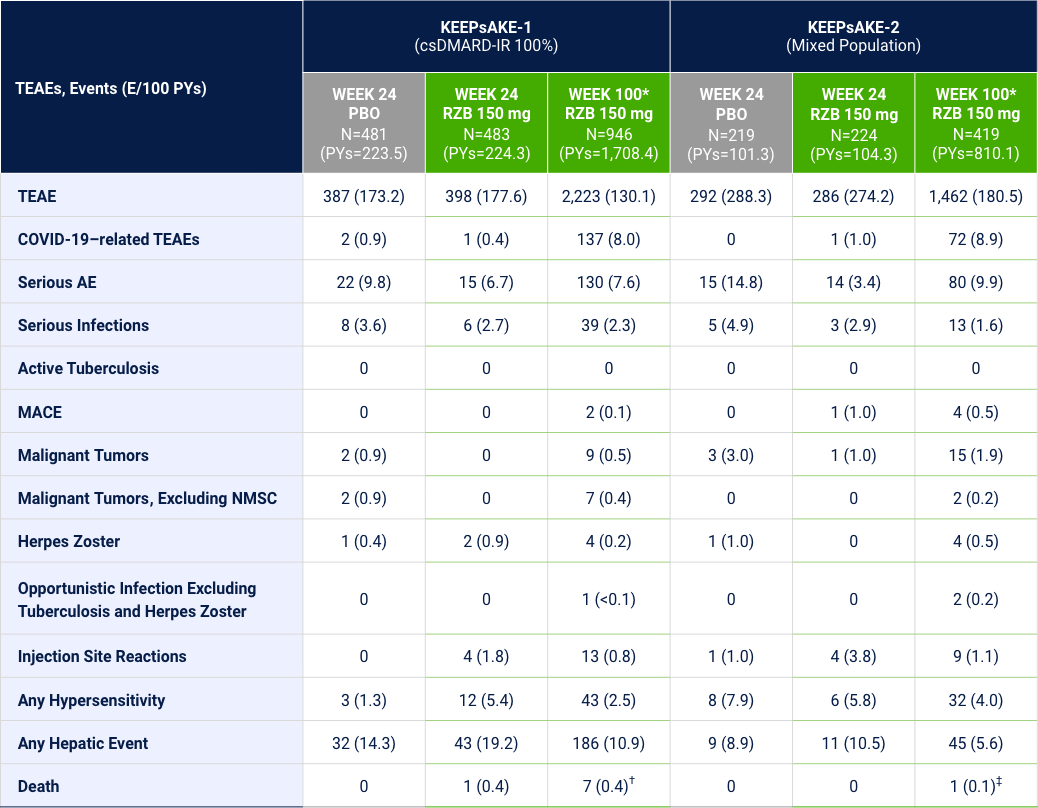
*Safety reported through data cutoff date (21 March 2022), which includes data through Week 100. Data are from any RZB 150-mg group that includes all patients who received RZB 150 mg. Including those who started on RZB 150 mg at randomization and who switched from placebo to RZB 150 mg after Week 24. †There were 6 patients with fatal treatment emergent events: death in 2 subjects was related to COVID 19; 1 subject died due to complications related to acute leukemia; 1 subject with anemia from diverticulosis died due multiorgan failure from septicemia as a complication from anastomosis surgery (left hemicolectomy surgery); 1 subject, 81 yrs of age with dementia, hospitalized for pneumonia, developed urosepsis and complications resulting in death; 1 subject hospitalized for anxiety and depression, 1 week after being discharged developed septicemia, nausea, vomiting, fever, and loss of appetite; death from an unknown cause (no autopsy) occurred 1 week later; Additionally, there was 1 subject died on day 363 (166 days after last dose) and the cause of death was reported as cardiorespiratory arrest. ‡There was 1 death with the event of coronary artery plaque rupture: subject had multiple risk factors such as obesity, long history of smoking, hypertension, hypercholesterolemia, and a family history of cardiovascular disease.
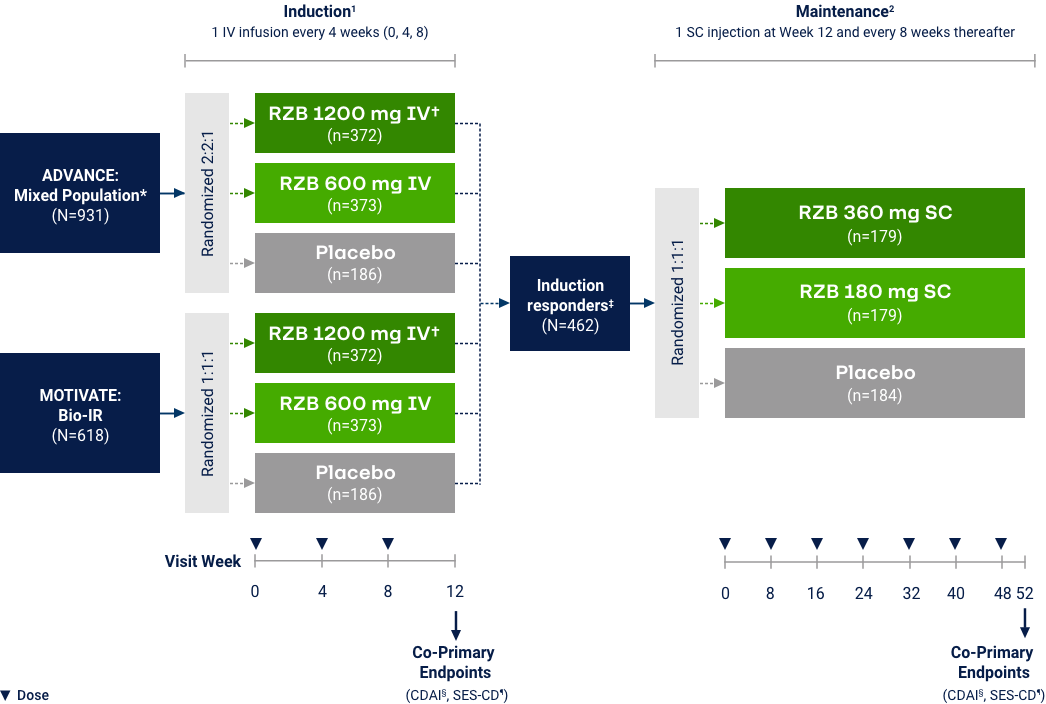
Crohn’s Disease
Co-primary Endpoints of Clinical Remission (CDAI < 150) at Weeks 12 and 525
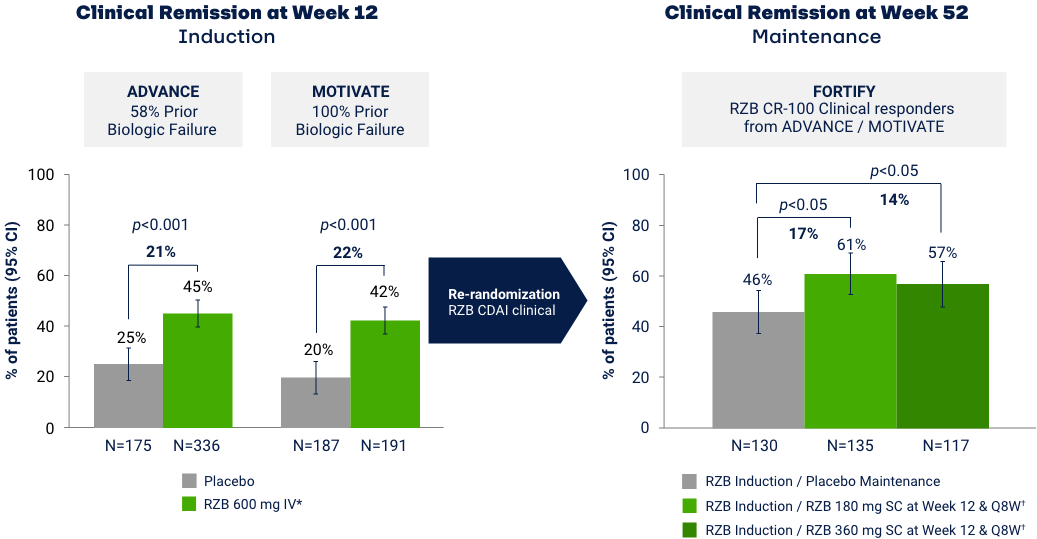
*RZB 600 mg as an intravenous infusion on Week 0, Week 4, and Week 8. †RZB 180 mg or 360 mg subcutaneously at Week 12 (Week 0 of FORTIFY) and every 8 weeks thereafter for up to an additional 52 weeks. Maintenance efficacy analysis based on re-randomization of RZB CDAI clinical responders (participants who received RZB induction therapy and achieved a reduction of
Co-primary Endpoints of Endoscopic Response at Weeks 12 and 525
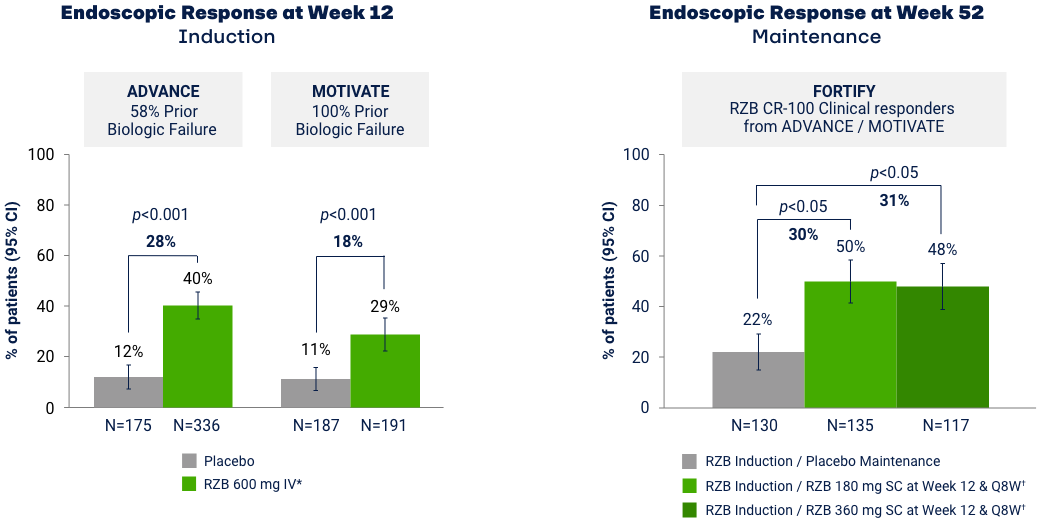
*RZB 600 mg as an intravenous infusion on Week 0, Week 4, and Week 8. †RZB 180 mg or 360 mg subcutaneously at Week 12 (Week 0 of FORTIFY) and every 8 weeks thereafter for up to an additional 52 weeks. Maintenance efficacy analysis based on re-randomization of RZB CDAI clinical responders (participants who received RZB induction therapy and achieved a reduction of
Endoscopic Remission: Ranked Secondary Endpoint at Weeks 12 and 52*
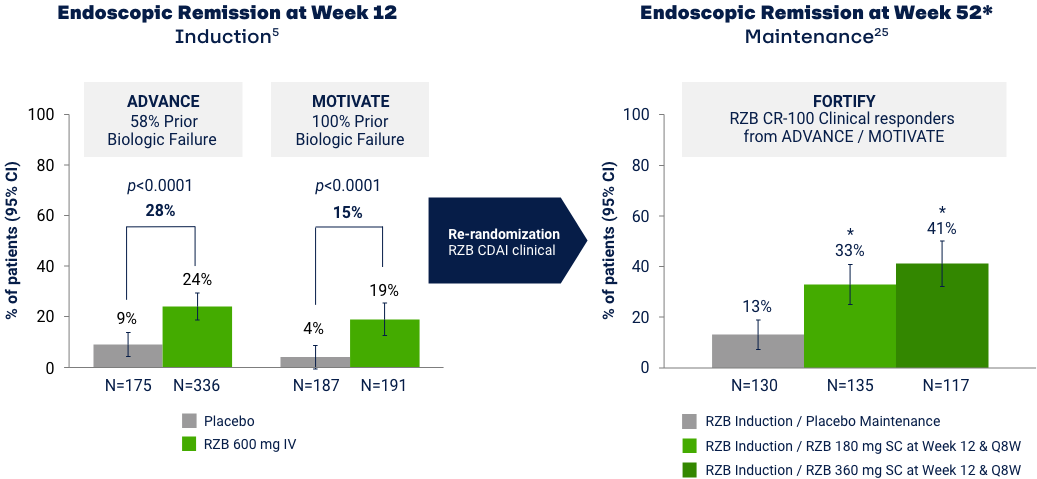
*The ranked secondary endpoint at Week 52 fell below the hierarchal testing procedure for statistical significance, thus this endpoint shown was not tested for multiplicity control and has a nominal p-value.255 Maintenance efficacy analysis based on re-randomization of RZB CDAI clinical responders (participants who received RZB induction therapy and achieved a reduction of CDAI > 100 points vs baseline). Error bars represent 95% CI. Endoscopic remission: SES-CD ≤ 4 and at least a 2-point reduction from baseline and no subscore greater than 1 in any individual variable, as scored by a central reviewer.9
Treatment-emergent Adverse Events of Special Interest26
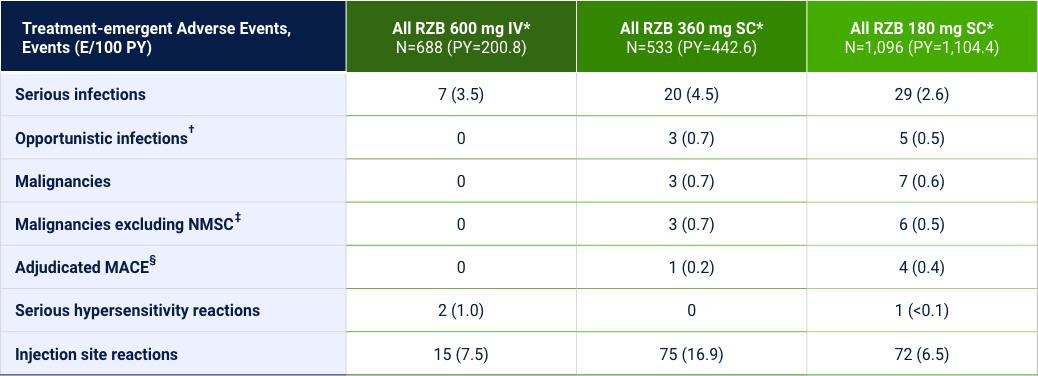
Hepatic events, events (E/100 PY): ¶All RZB 600 mg IV=21 (10.5); All RZB 180 mg SC=45 (4.1); All RZB 360 mg SC=18 (4.1).
Risankizumab may increase the risk of infection. Instruct patients to seek medical advice if signs and symptoms of clinically important infection occur. If such an infection develops, discontinue risankizumab until the infection resolves. Evaluate patients for tuberculosis prior to initiating treatment with risankizumab.5
*Safety analysis population: All patients that have received at least one dose of study drug from all Phase 2 and 3 CD studies and associated periods and substudies. †Opportunistic infections excluding tuberculosis and herpes zoster. ‡≥2 E/100 PY. §MACE, defined as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. ¶Hepatic events were defined by the following standardized MedDRA queries: “hepatic failure, fibrosis and cirrhosis and other liver damage-related conditions,” “hepatitis, non-infectious,” “cholestasis and jaundice of hepatic origin,” “liver related investigations, signs and symptoms,” and “liver-related coagulation and bleeding disturbances.”
Risankizumab-rzaa Indications and Important Safety Considerations
INDICATIONS
Risankizumab is indicated for the treatment of:
- Moderate to severe plaque psoriasis (Ps) in adults who are candidates for systemic therapy or phototherapy.
- Active psoriatic arthritis (PsA) in adults.
- Moderately to severely active Crohn’s disease (CD) in adults.
IMPORTANT SAFETY CONSIDERATIONS
Risankizumab is contraindicated in patients with a history of serious hypersensitivity reaction to risankizumab or any of the excipients. Serious hypersensitivity reactions, including anaphylaxis, may occur. If a serious hypersensitivity reaction occurs, discontinue risankizumab and initiate appropriate therapy immediately. Risankizumab may increase the risk of infections. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If such an infection develops, discontinue risankizumab until the infection resolves. Evaluate patients for tuberculosis infection prior to initiating treatment with risankizumab. Drug-induced liver injury/hepatotoxicity during induction treatment of CD has been reported. Monitor liver enzymes and bilirubin at baseline and during induction (12 weeks). Monitor thereafter according to routine patient management. Consider other treatment options in patients with evidence of liver cirrhosis. Interrupt treatment if drug-induced liver injury is suspected, until this diagnosis is excluded. Avoid use of live vaccines in patients treated with risankizumab. The most common adverse reactions (≥1%) reported during treatment for Ps and PsA are upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections. The most common adverse reactions (>3%) reported during treatment for CD are upper respiratory infections, headache, and arthralgia during induction dosing and arthralgia, abdominal pain, injection site reactions, anemia, pyrexia, back pain, arthropathy, and urinary tract infection during maintenance dosing.
Review risankizumab-rzaa full Prescribing Information for additional information, visit www.rxabbvie.com or contact AbbVie Medical Information at 1-800-633-9110.
ACR20=20% improvement on the American College of Rheumatology score; ADA=adalimumab; AE=adverse event; Bio=biologic; BMI=body mass index; BSA=body surface area; CDAI=Crohn’s Disease Activity Index; CFB=change from baseline; CI=confidence interval; csDMARD-IR=inadequate response to conventional synthetic disease-modifying antirheumatic drugs; FACIT=Functional Assessment of Chronic Illness Therapy; HAQ-DI=Health assessment questionnaire disability index; IGA=Investigator Global Assessment; IR=inadequate response; IV=intravenous; LDI=Leeds Dactylitis Index; LEI=Leeds Enthesitis Index; LS=least squares; MACE=major adverse cardiovascular event; MDA=minimal disease activity; mNAPSI=modified nail psoriasis activity index; mTSS=Modified Total Sharp Score; NMSC=nonmelanoma skin cancer; NRI=non-responder imputation; NRS=numerical rating scale; OLE=open label extension; PASI=psoriasis area and severity index; PBO=placebo; PGA-F=Patient Global Assessment Fingernail Psoriasis; PsA=psoriatic arthritis; PsO=Psoriasis; PtGA=Patient global assessment; PY=patient-year; Q8W=every 8 weeks; RZB=risankizumab; SC=subcutaneous; SEC=secukinumab; SES-CD=Simple Endoscopic Score in Crohn’s disease; SF-36 PCS=short-from 36 physical component score; sPGA=static Physician’s Global Assessment; TB=tuberculosis; TEAE=treatment-emergent adverse event; TNF=tumor necrosis factor; UPA=upadacitinib; UST=ustekinumab.
References: 1. Gordon KB et al. Lancet. 2018;392(10148):650-661. 2. Blauvelt A et al. JAMA Dermatol. 2020;156(6):649-658. 3. Östör A et al. Ann Rheum Dis. 2022;81(3):351-358. 4. Kristensen LE et al. Ann Rheum Dis. 2022;81(2):225-231. 5. SKYRIZI (risankizumab-rzaa) [package insert]. North Chicago, IL: AbbVie, Inc.; 2022. 6. ClinicalTrials.gov. NCT03398135. 7. ClinicalTrials.gov. NCT03398148. 8. Kristensen LE et al. EADV Virtual Congress 2022. Poster 3480. 9. D’Haens G et al. Lancet. 2022;399:2015-2030. 10. Papp KA et al. AAD 2023. Poster 43928. 11. Strober B et al. J Eur Acad Dermatol Venereol. 2020;34(12):2830-2838. 12. Reich K et al. Lancet. 2019;394(10198):576-586. 13. ClinicalTrials.gov. NCT04524611. 14. Gordon KB et al. Br J Dermatol. 2022:186(3):466-475. 15. Gordon KB et al. Poster presented at SDPA Conference 2021. 16. Gordon KB et al. EADV Virtual Congress 2022. Poster 1607. 17. Kristensen et al. Ann Rheum Dis. 2021;80:1315-1316. 18. Östör A et al. Ann Rheum Dis. 2021;80:138-139. 19. Coates LC et al. Ann Rheum Dis. 2010;69:48-53. 20. Kristensen LE et al. Poster presented at FC21 Conference. 21. Östör A et al. Poster presented at FC21 Conference. 22. Data on file, AbbVie Inc. ABVRRTl73417. 23. Kristensen LE et al. ACR Convergence 2022. Poster 2145. 24. Data on file, AbbVie Inc. ABVRRTl74973. 25. Data on file, AbbVie Inc. ABVRRTI74186. 26. Data on file, AbbVie Inc. ABVRRTI73682.